Through the integration of the initial equation, Expression E.2 is obtained.
2025 AIChE Annual Meeting
(187bf) Insight into Solvent Molecule Conformational Changes Via Density Contraction Differential Equation: Bridging Flory-Huggins Theory to Sanchez-Lacombe Equation of State.
Author
1.Introduction
It is a well-established fact that solvent molecules exhibit both translational and rotational movements, which have been extensively studied and documented [1]. While conformational changes of solvent molecules near polymer chains have not been studied, the conformational changes of polymer chains in the presence of solvent molecules are well-acknowledged and widely recognized [2] . A molecular theory of fluids outlined by Sanchez [3] Led to development of the Ising fluid model, which uses three parameters to describe a fluid [4]. This theory expands to fluid mixtures and results in four type of phase diagrams, plus one more type which can be obtained from the previous ones in the temperature-concentration plane constructed from spinodal inequality. This describes LCST behavior in all mixtures at temperatures near the spinodal Temperature [5]. The Sanchez-Lacombe equation of state (EOS) can effectively predict the behavior of polymer solutions, in addition to classical fluids, by minimizing the chemical potential to determine equilibrium conditions. This theory is a single-parameter (∆P*) model for binary mixtures. The parameter is determined using the heats of mixing at infinite dilution. A crucial relationship is used by Sanchez [6] to calculate the heat of mixing.
dρ ̅/dφ1 =ρ ̅2 ψT ̃P* β
In this relationship (Eq.1) ρ ̅ and T ̃ represent the reduced density and reduced temperature respectively. P* is the scale pressure, β is compressibility coefficient, φ1 is the close-packed volume fraction of the solvent and ψ represents the interactions between the polymer and the solvent. The term on the right side relates to the process of moving a solvent molecule from an area of high concentration to an area of lower concentration [6]. The significance of E. 1 lies in the concept of solvent density contraction when a small amount of polymer molecules is added. While the authors emphasize the importance of this relation, they do not convert the differential equation into an algebraic form.
2.Solving the differential equation
ρ ̅p-ρ ̅s= - ρ ̅s2 ψT ̃P* β
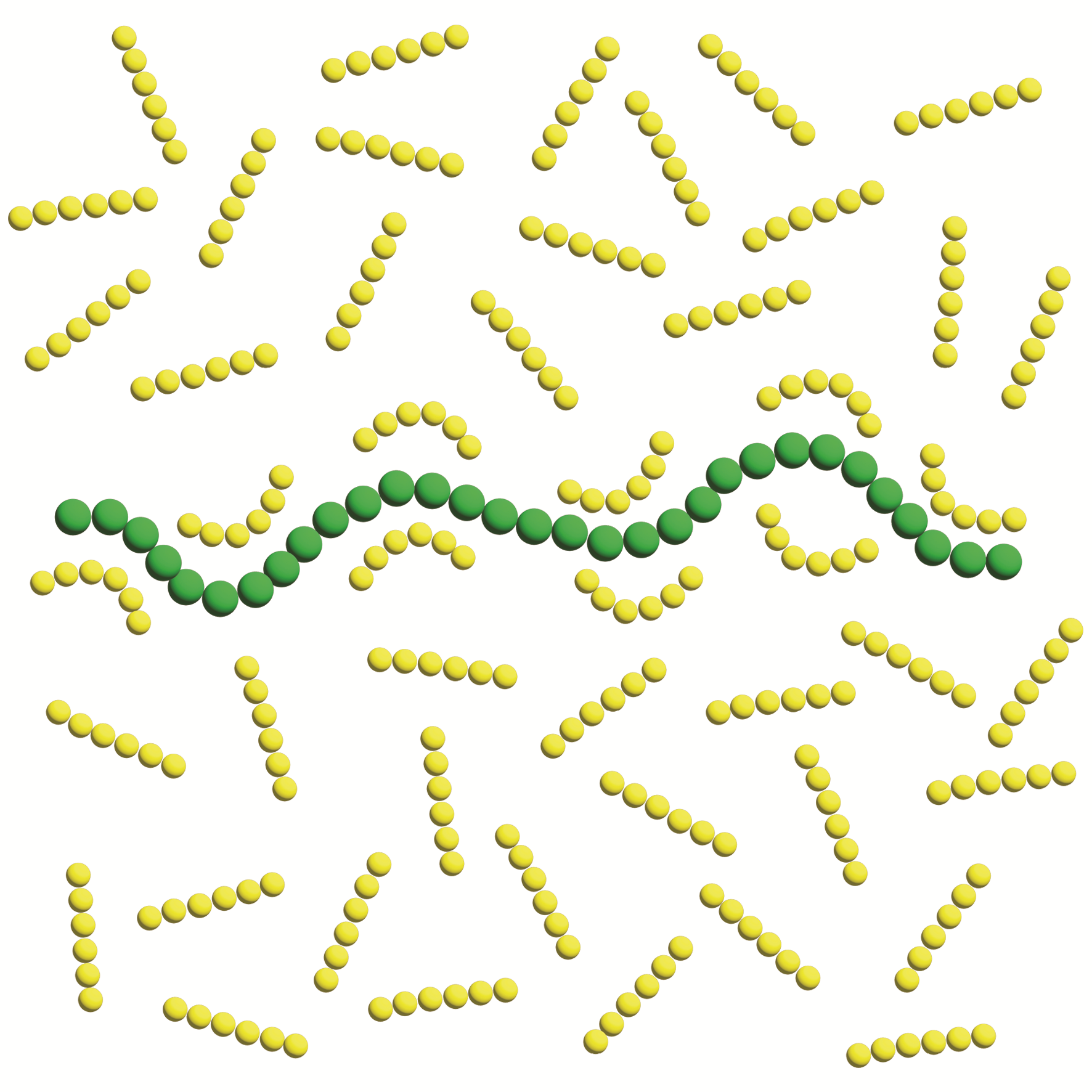
ρ ̅p and ρ ̅s represent the reduced density of polymer and solvent, respectively. It should be noted that the accuracy of Equation 2 depends on the size and structure of the solvent molecules. applying E. 2 to dilute polyisobutylene solutions for various solvents [6] demonstrates particularly precise agreement for cyclic structures. Cyclohexane exhibits the best agreement, while n-decane and n-octane also show good agreement. Circularity and flexibility are both crucial factors that contribute to greater compatibility with this equation, with circularity being more important. The comparison between cyclohexane and benzene highlights that the flexibility of cyclohexane, combined with its rounded structure, makes it more compatible with this equation. Regarding linear alkanes, their flexibility increases with length, so n-decane has the best compatibility. It is well-known that polymer chains conformational changes accour in the solvent [2] but as mentioned above flexibility of the solvent molecules and their circular structure is crucial for adopting the results with the contraction equation obtained from E. 1. So this suggests that solvent molecules also change their conformation near the polymer chains (Figure1).
As Furth states, “the arrangement of particles in a liquid has the character of a short-distance order” [7]. This order can be disturbed near polymer chains, leading to changes in the magnitude of order compared to pure solvent regions. Such disturbances can influence the combinatorial entropy component. The impact arises from new hypothetical entities formed by solvent molecules near the polymer chains, which are arranged as relatively long solvent molecules. This can lead to a reduction in the entropy change of polymer-solvent systems compared to regular solutions, which aligns well with previous findings. As Kumar states “the actual entropy change on mixing is found to be less than the predicted theoretical value because polymer molecules in dilute solution exist as isolated random coils whose sizes are a function of the molecular weight” [8]. So interaction parameter is a function of polymer chain length [2] [9]. It is noticeable that in the differential equation, ψ serves as a representative of the interaction parameter (χ). The average short-distance order of a pure solvent is significantly different from the short-distance order near the polymer chains. Therefore, in dilute solutions with isolated polymer chains, this can lead to a significant deviation from the Flory-Huggins theory. Using these results in the Flory-Huggins theory enables the identification of a close relationship with the Sanchez-Lacombe EOS, where a term replaces real pressure and can be understood as an apparent pressure arising from interactions between polymer chains and solvent molecules.
4.Conclusion
In this study, an important differential equation related to the density contraction of polymer solutions was integrated. It was demonstrated that the differential form of this equation can be simplified to an algebraic equation. By comparing the results, it was found that better agreement is achieved by including an additional coefficient beyond what was originally proposed. The findings suggest that the size and structure of solvent molecules can influence the outcomes, indicating conformational changes in solvent molecules. Furthermore, solvents near the polymer chain undergo short-distance order changes, introducing new hypothetical components that alter the entropy of the solution. These findings enable a simplified transition from the Flory-Huggins theory to the Sanchez-Lacombe equation of state (EOS).
References
1-R. D. O. a. F. D. B. B. NaNagara, "Mobility of toluene in polystyrene-toluene solutions: a NMR study," The Journal of Physical Chemistry, vol. 96, no. 15, pp. 6417-6423, 1992 https://doi.org/10.1021/j100194a058.
2-P. J. Flory, "Principles of Polymer Chemistry," Cornell University Press, Ithaca, NY, 1953.
3-I. C. S. a. R. H. Lacombe, "Theory of liquid–liquid and liquid–vapour equilibria," Nature, vol. 252, no. 5482, pp. 381-383, 1974. DOI: 10.1038/252381a0.
4-I. C. S. a. R. H. Lacombe, "An elementary molecular theory of classical fluids. Pure fluids," The Journal of Physical Chemistry, vol. 80, no. 21, pp. 2352-2362, 1976. DOI: 10.1021/j100562a008
5-R. H. L. a. I. C. Sanchez, "Statistical Thermodynamics of fluid Mixtures," The Journal of Physical Chemistry, vol. 80, no. 23, pp. 2568-2580, 1976. DOI: 10.1021/j100564a009
6-I. C. S. a. R. H. Lacombe, "Statistical Thermodynamics of Polymer Solutions," Macromolecules, vol. 11, no. 6, pp. 1145-1156, 1978. DOI.org/10.1021/ma60066a017.
7-R. Fürth, "On the theory of the liquid state: I. The statistical treatment of the thermodynamics of liquids by the theory of holes," Mathematical Proceedings of the Cambridge Philosophical Society, vol. 37, no. 03, pp. 252-275, 1941. DOI: 10.1017/S0305004100021745
8-R. K. G. Anil Kumar, "Fundamentals of Polymer Engineering," CRC press, 2018.
9-W. R. K. a. P. J. Flory, "Statistical Mechanics of Dilute Polymer Solutions. V. Evaluation of Thermodynamic Interaction Parameters from Dilute Solution Measurements1," Journal of the American Chemical Society, vol. 75, no. 21, pp. 5254-5259, 1953. DOI: 10.1021/ja01117a034.