2025 AIChE Annual Meeting
(236b) Inherent Dual-Functional Ca-Fe Materials Derived from Steel Slag for Integrated CO2 Capture and Utilization
Author
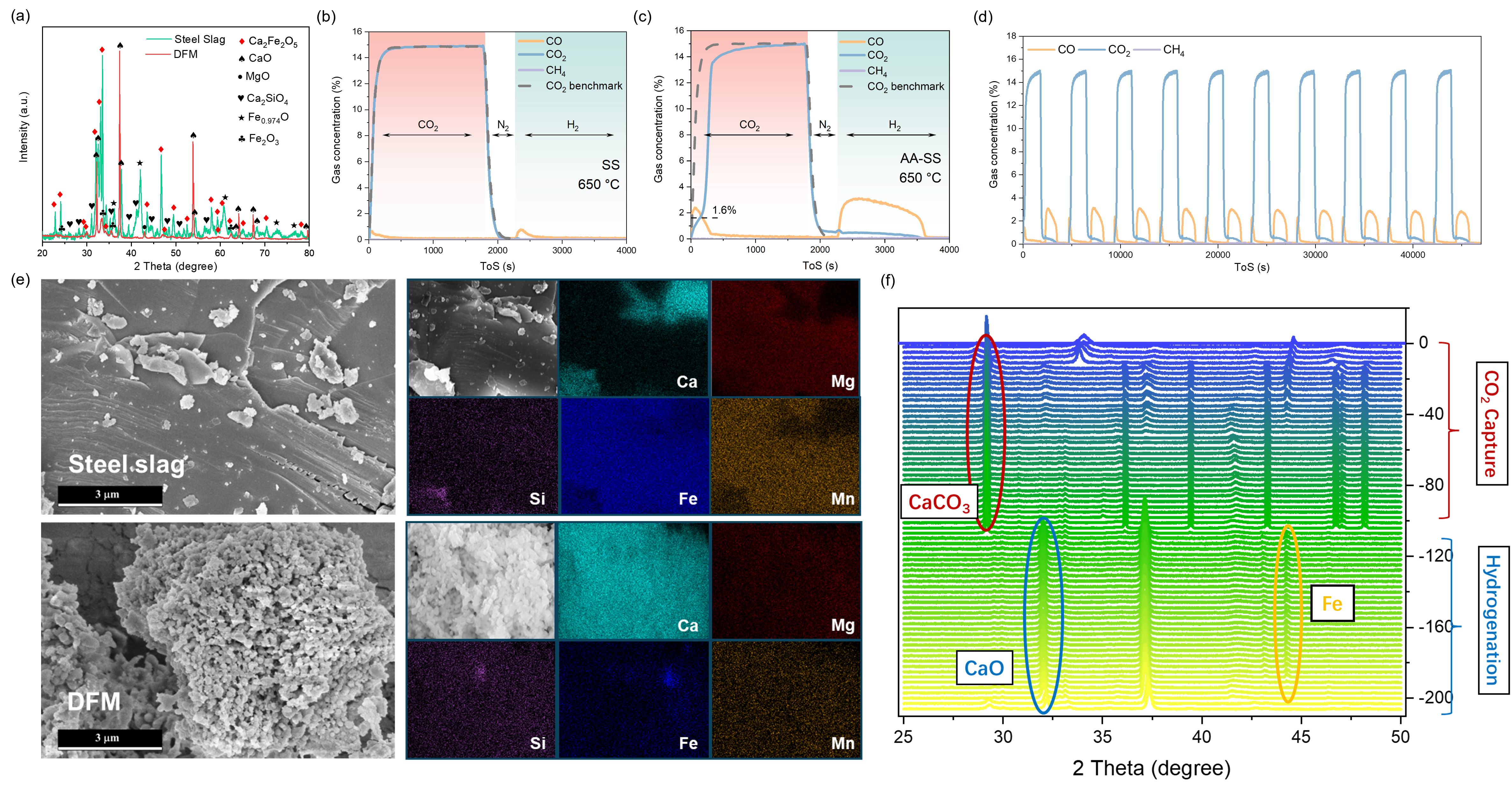
In contrast to traditional DFMs that rely on the addition of external catalytic materials, this study proposes DFMs derived from industrial steel slag, which inherently contains CaO as the CO2 sorbent and Fe as the catalytic component for the ICCU-RWGS reaction. The surface and bulk properties of these DFMs were thoroughly characterized using XRD, XPS, SEM-EDS, and TEM-EDS. The results showed that raw steel slag consists predominantly of inert phases such as Ca2Fe2O5 and Ca2SiO4, and features a dense, non-porous microstructure that restricts CO2 adsorption and in-situ conversion. However, after acetic acid pretreatment, the modified slag exhibited the formation of smaller pores and textural development, with active Ca present in the form of CaO. This transformation led to a remarkable 28-fold improvement in CO2 capture capacity, with the material retaining approximately 0.09 gCO2 g⁻¹ after 10 cycles and experiencing only a 20% capacity decline.
ICCU-RWGS tests conducted in a fixed-bed reactor demonstrated that the optimized steel slag-derived DFM achieved a CO2 uptake of 9.15 mmol g⁻¹, 87% CO2 conversion, a CO yield of 7.80 mmol g⁻¹, and 100% CO selectivity at 650 °C—far exceeding the performance of untreated steel slag (CO2 capacity < 1.0 mmol g⁻¹, CO yield = 0.73 mmol g⁻¹). In-situ XRD and FTIR analyses confirmed the presence of both self-sustaining CO2 sorption (CaO) and catalytic (Fe) functionalities, which are highly effective for the conversion of low-calorific-value waste gases. Overall, this work demonstrates the viability of converting steel industry waste into high-performance DFMs and presents a compelling pathway for sustainable carbon management through ICCU.