2025 AIChE Annual Meeting
(28b) The Influence of Polyelectrolytes on the Selective Hydrolysis of Hemicellulose from Bamboo Biomass Using Solid Acids Catalysts
However, fundamental understanding of the role of solid acids in converting lignocellulosic material is poorly understood, particularly due to the complexity of solid-solid interactions between the acid catalyst and plant polysaccharide components such as cellulose and hemicellulose. To better understand these interactions, DLVO theory has been applied to explain the formation of stable catalyst-polysaccharide colloids, as well as many of the challenges solid acids face during reaction. The net repulsive forces between catalysts and polysaccharides contribute to poor catalyst-substrate interaction, lower catalytic activity, and insufficient hydrothermal stability. The interactions between individual components found in plant-based biomass (lignin, cellulose and hemicellulose) and their own particular chemistries also make the compounds difficult to separate and depolymerize efficiently.
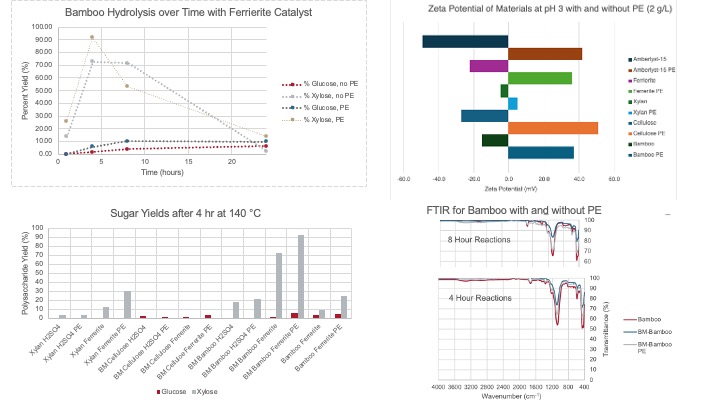
This work explores the influence of a common cationic polyelectrolyte, polyDADMAC, on the rate of ferrierite-catalyzed hydrolysis of bamboo biomass. The polyelectrolyte acts to neutralize surface charges of negative components (e.g. cellulose, xylan, bamboo, catalyst) and as a coagulant. Its addition to the reaction, polyDADMAC plays a key role in the selective hydrolysis of xylan, a main component in hemicellulose, into xylose, a process that can make cellulose and lignin more available for further reactions.
A xylose yield of 92% is found at temperatures of 140 °C and 4 hours for ball-milled bamboo. To better understand the relative reaction rates with the addition of both solid acids and polyelectrolytes, a kinetic study is performed and compared to the xylose and glucose yields found from a traditional homogenous acid catalyst (H2SO4). HPLC was used to quantify both sugar products and other byproducts (HMF, furfural), while gravimetric analysis characterized leftover solids. The zeta potential of catalysts, polysaccharides and whole bamboo were used to determine changes in the charge with and without the addition of polyDADMAC. FTIR was used to understand polysaccharide breakdown. The pH was monitored to understand acid leaching. Ultimately, the addition of polyDADMAC allowed for a selective hydrolysis of xylan in bamboo biomass using a ferrierite solid acid catalyst.
[1] Langholtz, Matthew H., et al. "2023 Billion-Ton Report: An Assessment of US Renewable Carbon Resources." (2024).