2025 AIChE Annual Meeting
(117h) Improved Study of the Oxidative Coupling of Methane (OCM) at Elevated Pressure: Investigating Gas Phase OCM Using Calculated Reactor Bed Void Volume
Authors
Despite the need for commercialization, limited work has been done so far at elevated pressures. This study aims to demonstrate a mechanistic approach in studying the gas phase OCM with dependency on bed void volume and addresses the synergy between gas-phase and surface catalytic OCM at elevated pressures. Different reactor setups were designed to give varying void volume and allow the study of gas phase OCM both in catalytic and non-catalytic conditions.
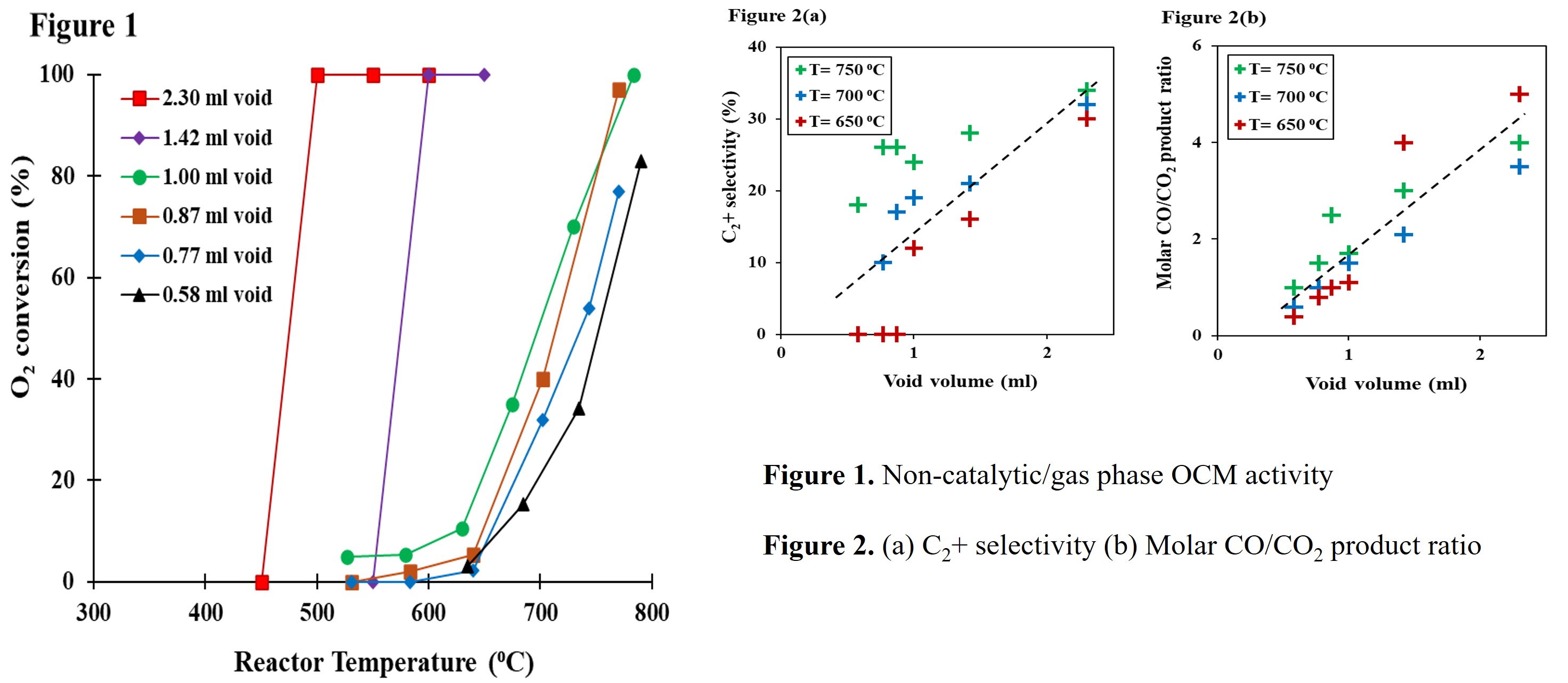
A recent study has shown possible improvement in OCM performance with particle bed characteristics, such as flow and heat distribution in bed particle clusters. In this case, carrying out non-catalytic OCM reaction in reactors filled with inert material of different sizes and void volume clearly indicates a linear dependency between gas phase activity and void volume. The relationship between void volume and OCM gas phase activity is seen in figure 1, using O2 conversion as the metric for extent of activity. In addition to the activity plot, figure 2(a) and (b) shows coupling activity increasing and formation of more CO products w.r.t void volume. With this knowledge, further studies were carried out with the catalyst loaded in different sizes and void volume- which allowed the study of gas phase effect in a typical OCM catalytic reaction.