2025 AIChE Annual Meeting
(355a) Impacts of Beta-Sheet Crystallinity on the Mechanical Properties of Chemically and Physically Crosslinked Silk Fibroin Hydrogels
Authors
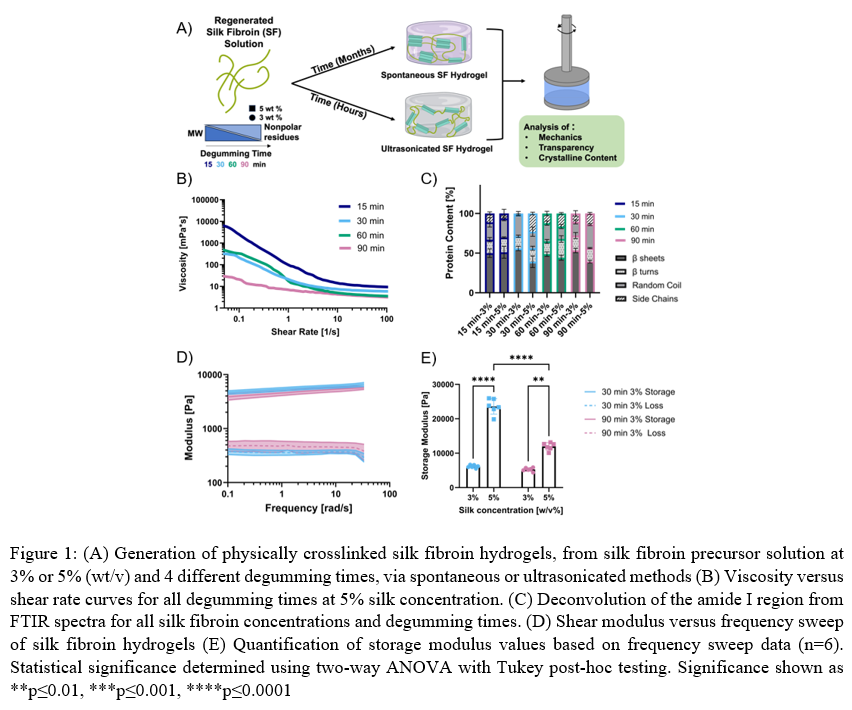
Methods: Silk fibroin solution was isolated from the cocoons of Bombyx mori silkworms.1 Briefly, cocoons were cut into small pieces and boiled (degummed) for 15, 30, 60, or 90 minutes in sodium carbonate solution and the resulting silk fibroin mat was left to dry. The mat was then solubilized in 9.3 M lithium bromide. After undergoing dialysis, the silk was diluted with ultrapure water to 3% or 5% (wt/v) silk fibroin solution. Gel electrophoresis (SDS-PAGE) and HPLC were used to assess molecular weight and amino acid content, respectively. Two methods of physically crosslinked hydrogels were used. Spontaneous physically crosslinked hydrogels were formed by leaving the silk solution to spontaneously form hydrogels over time though beta-sheet formation. Ultrasonicated physically crosslinked hydrogels were formed by sonicating silk fibroin solution for 15 seconds at 15% amplitude. Silk fibroin was chemically modified to enable photocrosslinking, through the azo-based amino tyrosine premodification followed by the addition of a norbornene.9, 10 Resulting physical crosslinking (beta-sheet content) was assessed over time through Fourier transform infrared (FTIR) spectroscopy and optical transparency. Mechanical properties of silk fibroin precursor solutions and final hydrogels were assessed via rheology and dynamic mechanical assessment though shear measurements over time to obtain viscosity and modulus values.
Results: Increasing the degumming time of the polymer extraction decreases the molecular weight due to denaturation of the polymer during boiling.11 During the degumming process, thermal degradation results in changes to the distribution of molecular weights as well as primary amino acid structures. We hypothesized that the shorter degumming times and higher polymer concentration would lead to more beta-sheet structures and polymer interactions, which would result in larger mechanical property values. Traditional non-crystalline polymer trends predict that the larger molecular weight conditions (15-minute degumming) will have the highest viscosity, and viscosity will decrease as degumming time increases. However, this trend did not hold for all SF solutions due in part to differences in inter- and intra-molecular interactions from differences in polymer entanglement of the semi-crystalline polymer. We did find that all conditions show shear thinning behavior over low (0.1 to 100 sec-1) shear rates. This phenomenon also had an impact on beta-sheet structure formation found via deconvolution of FTIR spectra. We hypothesized that the higher molecular weight hydrogels would result in more beta-sheet structures. However, we found that the physical crosslinking method had a larger impact on mechanical properties and that gelation by sonication generates lower initial beta-sheet content. However, over time, the beta-sheet content increases due to spontaneous formation still occurring. Results suggest that the time scale of hydrogel formation in sonicated hydrogels has too short of a gelation time for the beta-sheet structures to organize in their lowest energy state, whereas the spontaneous hydrogel has longer gelation times allowing the polymer chains to rearrange and form more organized secondary structures. Results confirm that SF molecular weight has the greatest impact on viscosity and the greatest impact on physical crosslinking dynamic moduli.
Similarly, preliminary work using chemically modified silk fibroin was used to form hydrogels via photopolymerization, yet physical crosslinks still occurred over time. Most importantly, the dynamics of these changes (timescale) were substantially different from the timescales measured with ultrasonicated hydrogels. Modulating temporal control through chemical crosslinking methods allows for alterations in gelation time and the resulting beta-sheet content over different time scales through more the addition of more flexible chemical linkers. Understanding the spontaneous beta-sheet formation in SF hydrogels enables temporal control over mechanical properties for future hydrogel applications, such as 3D printing or soft tissue engineering, including in vitro tumor modeling.
References:
- Rockwood DN, Preda RC, Yücel T, Wang X, Lovett ML, Kaplan DL. Materials fabrication from Bombyx mori silk fibroin. Nature Protocols. 2011;6(10):1612-31. doi: 10.1038/nprot.2011.379.
- Stoppel WL, Gao AE, Greaney AM, Partlow BP, Bretherton RC, Kaplan DL, Black LD. Elastic, silk-cardiac extracellular matrix hydrogels exhibit time-dependent stiffening that modulates cardiac fibroblast response. Journal of Biomedical Materials Research Part A. 2016;104(12):3058-72. doi: 10.1002/jbm.a.35850.
- Barroso IA, Man K, Villapun VM, Cox SC, Ghag AK. Methacrylated Silk Fibroin Hydrogels: pH as a Tool to Control Functionality. ACS Biomaterials Science & Engineering. 2021;7(10):4779-91. doi: 10.1021/acsbiomaterials.1c00791.
- Matsumoto A, Chen J, Collette AL, Kim U-J, Altman GH, Cebe P, Kaplan DL. Mechanisms of Silk Fibroin Sol−Gel Transitions. The Journal of Physical Chemistry B. 2006;110(43):21630-8. doi: 10.1021/jp056350v.
- Matsumoto A, Lindsay A, Abedian B, Kaplan DL. Silk Fibroin Solution Properties Related to Assembly and Structure. Macromolecular Bioscience. 2008;8(11):1006-18. doi: 10.1002/mabi.200800020.
- M. O. Pacheco ELA, H. K. Bagnis, I. K. Gerzenshtein, T. D. Truong, W. L. Stoppel. Self-assembled hydrogel properties demonstrate the dual role of boiling in silk fibroin processing: molecular weight reduction and enrichment of beta-sheet forming residues. ACS Biomacromolecules. 2025.
- Major G, Ahn M, Cho W-W, Santos M, Wise J, Phillips E, Wise SG, Jang J, Rnjak-Kovacina J, Woodfield T, Lim KS. Programming temporal stiffness cues within extracellular matrix hydrogels for modelling cancer niches. Materials Today Bio. 2024;25:101004. doi: https://doi.org/10.1016/j.mtbio.2024.101004.
- Guvendiren M, Burdick JA. Stiffening hydrogels to probe short- and long-term cellular responses to dynamic mechanics. Nature Communications. 2012;3(1):792. doi: 10.1038/ncomms1792.
- Murphy AR, John PS, Kaplan DL. Modification of silk fibroin using diazonium coupling chemistry and the effects on hMSC proliferation and differentiation. Biomaterials. 2008;29(19):2829-38. doi: 10.1016/j.biomaterials.2008.03.039.
- Hausken KG, Frevol RL, Dowdle KP, Young AN, Talusig JM, Holbrook CC, Rubin BK, Murphy AR. Quantitative Functionalization of the Tyrosine Residues in Silk Fibroin through an Amino‐Tyrosine Intermediate. Macromolecular Chemistry and Physics. 2022;223(17):2200119. doi: 10.1002/macp.202200119.
- Pacheco MO, Lutz HM, Armada J, Davies N, Gerzenshtein IK, Cakley AS, Spiess BD, Stoppel WL. Silk Fibroin Particles as Carriers in the Development of All-Natural Hemoglobin-Based Oxygen Carriers (HBOCs). 2023.