2025 AIChE Annual Meeting
(229c) Impact of Various Carriers and Oxygen Vacancy on Na-Based DAC Sorbents
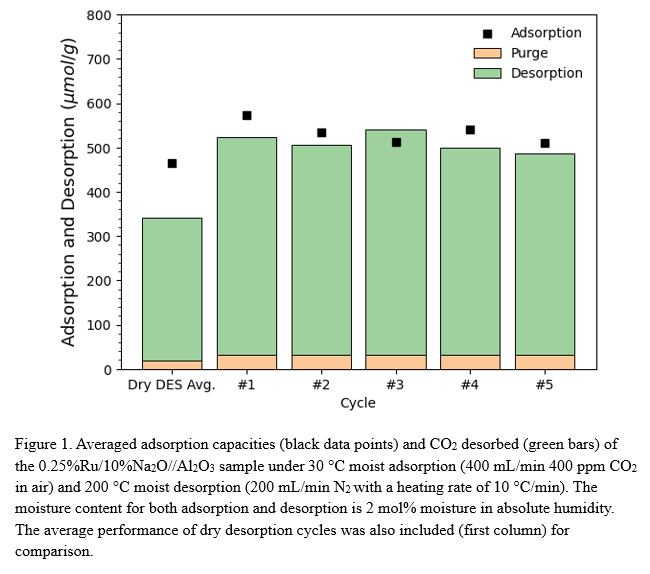
Previously, we demonstrated that Na-based sorbents composed of Ru/Na2O//Al2O3 exhibit exceptional adsorption and regeneration performance under realistic DAC conditions with varying temperature and moisture conditions [1]. In recent tests, we evaluated a sorbent containing 0.25% Ru intimately dispersed with 10% Na2O on an Al2O3 carrier. The figure illustrates 5 cycles of adsorption of CO2 (black data points) with simulated ambient air of 400ppm CO2 in air and 2 mol% humidity at 30 °C. Purge N2 is then introduced to desorb captured CO2 at 200 °C with a heating rate of 5 °C/min (green columns). It achieved an average adsorption capacity of 534.52 μmol/g and CO2 desorption of 479.49 μmol/g, with a remarkable regeneration efficiency of approximately 90%. It outperformed the results with dry desorption (first column), which has a slightly lower ADS/DES performance with a regeneration efficiency of roughly 70% [2].
Given the performance of the current material, we extended the scope of this study by investigating additional carrier materials, including TiO2, CeO2, and ZrO2. Many of these metal oxides can form oxygen vacancies, which can potentially improve the adsorption and desorption behavior of our sorbent. Theoretically, oxygen vacancies can activate CO2 due to the unsaturated chemical bond in the metal oxide and unshared electron pairs of CO2 [3]. It is also reported that additional oxygen vacancies can be generated by thermal desorption of lattice oxygen or pretreatment by reactive molecules like H2 and CO [3][4], which is similar to the proprietary pretreatment method employed for our material. Therefore, incorporating these alternative metal oxides as carrier materials is likely to further enhance the performance of the sorbent.
Reference:
[1] Jeong-Potter, C.; Abdallah, M.; Sanderson, C.; Goldman, M.; Gupta, R.; Farrauto, R. Dual Function Materials (Ru+Na2O/Al2O3) for Direct Air Capture of CO2 and in Situ Catalytic Methanation: The Impact of Realistic Ambient Conditions. Applied Catalysis B: Environmental 2022, 307, 120990.
[2] Kim, S.; Lin, X.; Farrauto, R. J. Effect of Moisture (2 Mol%) on CO2 Enhanced Desorption from Nano-Dispersed Na2O/Al2O3 for Direct Air Capture. Chemical Engineering Journal 2024, 499, 156238.
[3] Etim U.J.; Zhang C.; Zhong Z. Impacts of the Catalyst Structures on CO2 Activation on Catalyst Surfaces. Nanomaterials (Basel). 2021, 11(12):3265.
[4] Chen, S.; Cao, T.; Gao, Y.; Li, D.; Xiong, F.; Huang, W. Probing surface structures of CeO2, TiO2, and Cu2O nanocrystals with CO and CO2 chemisorption. J. Phys. Chem. C 2016, 120, 21472–21485