2025 AIChE Annual Meeting
(542h) Impact of Menstrual Product Derived Nanoparticles on Vaginal Mucosal Barrier Integrity
Authors
Menstrual products have recently been shown to release up to 7 billion particles per single use, yet the impact of these particles on mucosal barriers remains poorly understood. The vaginal epithelium functions as both a physical and immunological barrier, protecting against dehydration, toxins, and pathogens. With over 1.8 billion menstruating individuals globally and a significant proportion using menstrual hygiene products, understanding how nanoparticles interact with the vaginal mucosal barrier is essential for public health and product safety. Although environmental nanoparticles have been linked to adverse biological effects, little is known about how menstrual product-derived particles affect the vaginal epithelium or its barrier function. In biological environments, nanoparticles rapidly acquire a protein corona, which can alter surface properties and enhance cellular interactions. Thus, we hypothesize that menstrual blood proteins adsorb to nanoparticles, forming a biological corona that facilitates their penetration and disruption of the vaginal epithelial barrier.
Methods
Human vaginal epithelial cells (VEC) were seeded at a density of 2.8×10⁵ cells/cm² on 96-well transwell plates (8 µm pore size) to establish a physiologically relevant barrier. Barrier integrity was assessed through transepithelial electrical resistance (TEER) measurements and passive diffusion of 3,000 MW fluorescently labeled dextran particles. Polystyrene nanoparticles were introduced at 25 particles per cell, reflecting concentrations relevant to menstrual product exposure. To examine the influence of menstrual blood proteins on nanoparticle interaction with the epithelial barrier, a subset of nanoparticles was incubated in menstrual blood (MB) to allow protein adsorption, then washed to remove unbound material. Comparisons were made between control (no particles), uncoated nanoparticles, and MB-coated nanoparticles to evaluate changes in barrier integrity.
Results
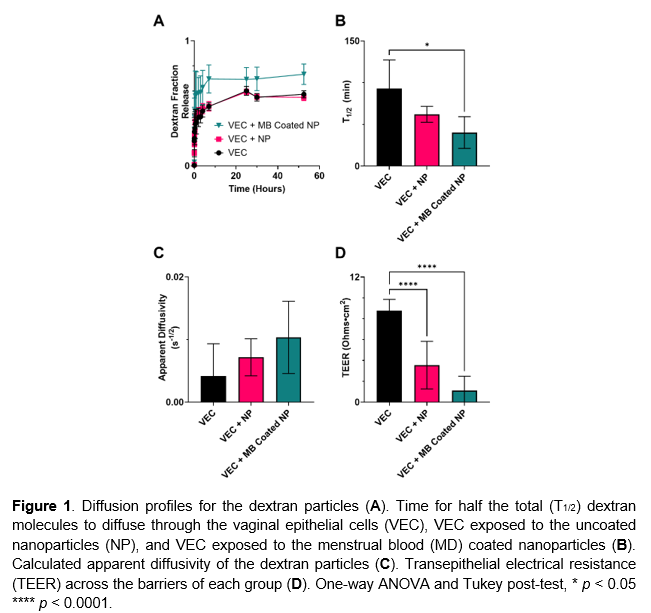
The time required for half of the dextran particles to diffuse across the epithelial layer (T1/2) was shortest in VECs exposed to MB-coated nanoparticles, followed by uncoated nanoparticles, and longest in the control group with no nanoparticle exposure (Fig. 1A). Diffusion profiles (Fig. 1B) and calculated apparent diffusivity values (Fig. 1C) followed similar trends, with the MB-coated group showing the highest transport rates. Although diffusivity differences were not statistically significant, a consistent trend was observed. TEER measurements (Fig. 1D) confirmed these results, with the highest barrier integrity in the control group and significantly reduced integrity in both nanoparticle-exposed groups, especially those exposed to MB-coated particles.
Conclusion
These data demonstrate that nanoparticles from tampons can compromise mucosal barrier integrity by increasing epithelial permeability. Notably, this was enhanced in the presence of menstrual blood, indicating that a protein coating is facilitating nanoparticle penetration and disruption of the vaginal epithelial barrier. The observed reduction in vaginal epithelial barrier function following nanoparticle exposure raises important concerns about increased vulnerability to pathogen invasion. Given the widespread use of menstrual products that may shed nanoparticles, these results underscore the need for greater scrutiny of product safety and the long-term implications for reproductive health.