2025 AIChE Annual Meeting
(451f) Hydrogen Production By E-Field Enhanced Thermo-Catalytic Decomposition of Natural Gas Using Carbon Catalysts
TCD, an alternative energy technology produces (blue) hydrogen by decarbonizing fossil fuels such as natural gas or coal-derived volatiles or heavy hydrocarbons, providing a bridge to the hydrogen economy. A limitation is the deactivation of the carbon catalyst, more specifically the depositing carbon exhibits lessening activity with reaction duration. Complementing TCD is regeneration. Partial oxidation by CO2 creates new active sites, thereby renewing carbon catalyst activity. Partial gasification of deposited carbon by H2O (generating H2) establishes the baseline for coal gasification. Neither TCD nor carbon oxidation have been tested under an E-field for change in activation energy or mechanism. For both reactions, an imposed electric field offers potential to maintain and potentially increase the reaction rate; occurring either by an increase in active site number or shift in their energy level. (Correspondingly, the associated activation energy for elementary reaction step(s) would also change).
Hypothesized is that an applied E-field changes the reaction mechanism, manifested by activation energy and kinetics of deposition and regeneration. Two E-field configurations, perpendicular imposing only voltage stress versus parallel, imposing current stress will be tested. Active site quantification and kinetic rate measurements will be performed across a temperature-time matrix. Analytical techniques include XPS for quantifying active site number via chemisorbed oxygen, resolved by functional group and Raman for comparative defects. Kinetic rates will be based upon gravimetric measurement of deposited carbon in TCD, measurement of CO concentration in regeneration by CO2 or H2 concentration in regeneration with H2O. Activation energies will be extracted and evaluated for steadiness or change. Active site and kinetic dependence upon reactive gases and their concentrations will be mapped parametrically as function of applied E-field strength, polarity, direction and frequency. Therein changes in rates may be resolved by active site number or activation energy under E-field action. Carbon catalyst metrics of activity and stability will be assessed in TCD, and after regeneration to assess effectiveness.
Experimental Progress
To illustrate the capabilities of Joule-based heating for TCD and RGN, a SS mesh was used as a deposition substrate and subsequently the support for oxidation of the (previously deposited) carbon. The mesh is a particularly flexible form for packed beds or similar configurations. We have previously tested other porous metal forms and found the mesh to be resilient and reusable.
Traditional packed beds, particularly as reported in the literature for TCD involve various carbon forms, including carbon blacks, carbon nanotubes, engineered porous carbons or coal chars. Generally, a distributed inert filler is needed to increase bed permeability and to mitigate the tendency for “bed packing” under reaction conditions. However, TCD is unique in that the bed is not a normal heterogenous catalyst conforming to the Langmuir-Hinshelwood mechanism. Instead, solid carbon is deposited. Coking is the desired reaction outcome forming solid carbon. Ideally TCD would be autocatalytic, but across a vast array of carbon materials and reaction conditions, the rate decreases over time, necessitating a) fresh catalyst or b) regeneration of existing catalyst, partially or fully covered by deposited carbon.
The screen as a prototype for other planned bed configurations would allow carbon removal and / or regeneration in situ. The latter could readily be accomplished using a packed bed arrangement wherein deposited carbon is only partially oxidized, to regenerate active sites while the majority of the deposited carbon remains untouched, thereby largely preserving the carbon capture advantage of TCD and, uniquely the benefit of carbon in solid form as opposed to CO2.
In such a combined TCD / RGN system, a swing-bed system would be implemented wherein each bed would produce concentrated streams of H2 or CO, respectively. With each product stream isolated, no separation or purification stages would be needed as in wet or dry reforming processes. As the components of syn-gas, the separate streams could be combined in desired proportions for fuel and/or chemical synthesis.
For the dual goals of process intensification and utilizing the unique features of electric fields for modifying energy barriers and corresponding reaction rates, Joule-based heating is being tested. For purposes of illustration the mesh offers resiliency and higher porosity and permeability than any traditional packed bed. In particular the TCD carbon fills any bed configuration requiring innovations to traditional bed configurations and operation.
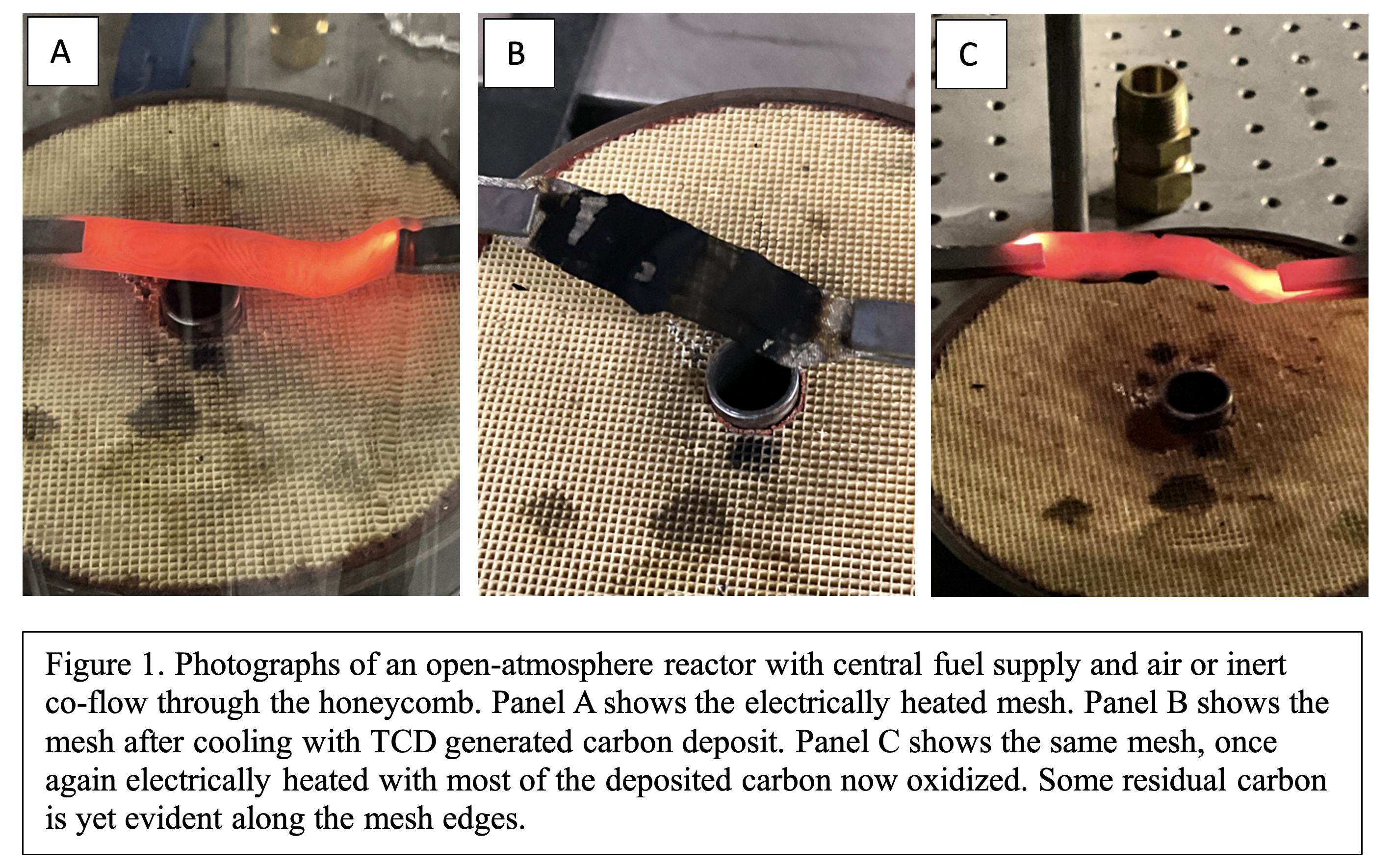
Figure 1 shows the apparatus used to provide controlled gas environments for testing the electrical heating of the SS mesh. A cordierite honeycomb provided laminar flow of inert or air for TCD or RGN reactions respectively. For TCD, the hydrocarbon was supplied through the central brass tube, above which the SS mesh was centered.
Panel A in Fig. 1 also shows the SS mesh under Joule-based heating with a pyrometric temperature estimate of 800 C. The “A-frame” shape was chosen to provide increased gas-surface contact time. Panel B in Fig. 2 shows the same mesh, post-heating, now covered the TCD generated carbon. Discerning whether the mesh visibly darkened during exposure to ethylene (a greenhouse gas) while heated was challenging; the radiant emission rendered small contrast between mesh and depositing carbon. (The deposited carbon was radiating as well as the mesh but with the higher emissivity of carbon). Upon cooling, the deposited carbon was evident, as shown. This image represents the first known demonstration of TCD under Joule-based heating.
To demonstrate RGN (and more generally, gasification of carbon) under Joule-based heating, the same mesh now with TCD carbon was electrically heated while exposed to an air flow. Panel C in Fig. 3 shows the mesh, electrically heating, with the deposited carbon nearly fully oxidized. Uniform oxidation was difficult given that the deposited carbon itself varied in amount across the mesh. Nonetheless oxidation could be regulated by current and resulting mesh temperature. We note that under this high oxygen condition, self-heating of the carbon by rapid oxidation likely occurred. Vitiated environments or using H2O or CO2 as oxidants would enable greater control of the reaction kinetics. This image represents the first known demonstration of carbon gasification under Joule-based heating.
Future Directions
Measurement of active sites and predictions by simulations will provide mechanistic insights for carbon surface reactions relevant to both TCD and regeneration reactions. Beyond the relation between reaction rate and active sites, the primary question of whether the E-field affects number and/or type of active site will be addressed.
Acknowledgement:
This material is based upon work supported by the Department of Energy under Award Number DE-FE0032070.