2025 AIChE Annual Meeting
(483a) A High-Throughput Platform for Investigating Transport of Intrathecally Injected Nanoparticles
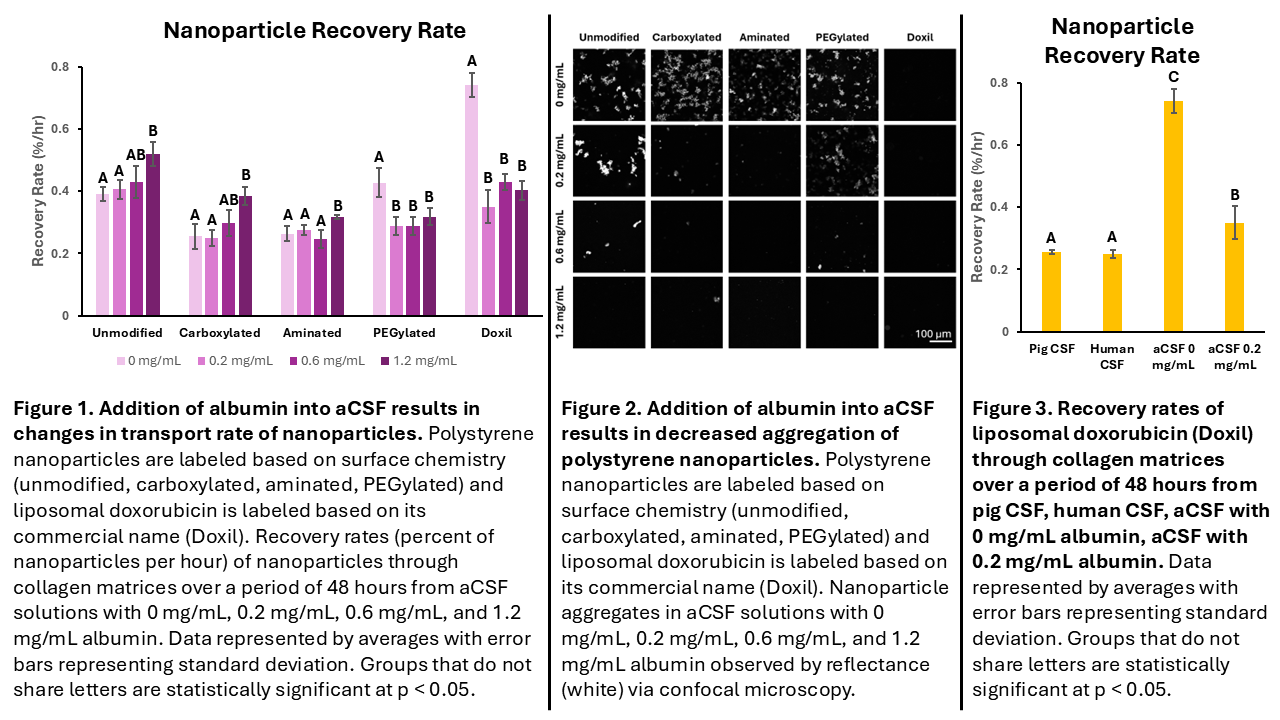
In this work, we investigate how albumin in artificial CSF (aCSF) alters the transport of nanoparticles with various surface chemistries through pia mater using a modified Transwell recovery assay. This model consists of two layers: 1) a solution of nanoparticles and aCSF, mimicking intrathecal injection microenvironment and 2) a pia mater mimic. Due to the high collagen I content in pia mater, a collagen I hydrogel was utilized as our model pia mater. The human proteome consists of 60% albumin by volume1; however, the albumin concentration in CSF is elevated in patients with neurological diseases. By varying the amount of albumin in aCSF, we evaluate how albumin biocorona, and thus disease state, impacts nanoparticle transport. The modified Transwell model allows us to evaluate the transport rate of nanoparticles through collagen from aCSF, modeling transport through pia mater from the injection microenviroment. When adding albumin into the aCSF solution, nanoparticle transport is impacted differentially depending on the nanoparticle surface chemistry. There was an increase in transport of unmodified, carboxylated, and aminated nanoparticles with an increase in albumin (Figure 1) due to decreased aggregation of nanoparticles in aCSF (Figure 2). PEGylated nanoparticles, such as PEGylated polystyrene and PEGylated liposomal doxorubicin (Doxil), see a rapid decline in their ability to traverse through collagen with albumin (Figure 1). Nanoparticle transport is decreased with albumin for PEGylated and Doxil nanoparticles due to increased interactions between nanoparticle and collagen, causing nanoparticles to traverse slower through the collagen matrix.
The results indicate that nanoparticle surface chemistry and nanoparticle albumin biocorona are key factors that impact how a nanoparticle can traverse across collagenous barriers, such as pia mater. Additionally, a comparison to pig and human CSF was performed, allowing us to observe that nanoparticle transport through CSF is modeled better with aCSF containing albumin than aCSF without albumin (Figure 3). Diffusion of nanoparticles is dependent on the interactions between nanoparticle surface chemistry, albumin in the biocorona, and collagen in the extracellular matrix. Taken altogether, these results illustrate how this platform can be utilized to investigate the effects of nanoparticle interactions within the injection environment, identify extracellular barriers to nanoparticle transport, and screen nanoparticle formulations to find formulations that will traverse into the drug delivery site.
1 Roche, S., Gabelle, A. & Lehmann, S. Clinical proteomics of the cerebrospinal fluid: Towards the discovery of new biomarkers. PROTEOMICS – Clinical Applications 2, 428–436 (2008).