2025 AIChE Annual Meeting
(676c) High-Pressure Electrochemical CO2 Capture and Reduction to Formic Acid
Authors
Shaoyun Hao, Zhejiang University
Malik Paulino, Rice University
Valery Okatenko, Rice University
Junwei Zhang, Rice University
Haotian Wang, Rice University
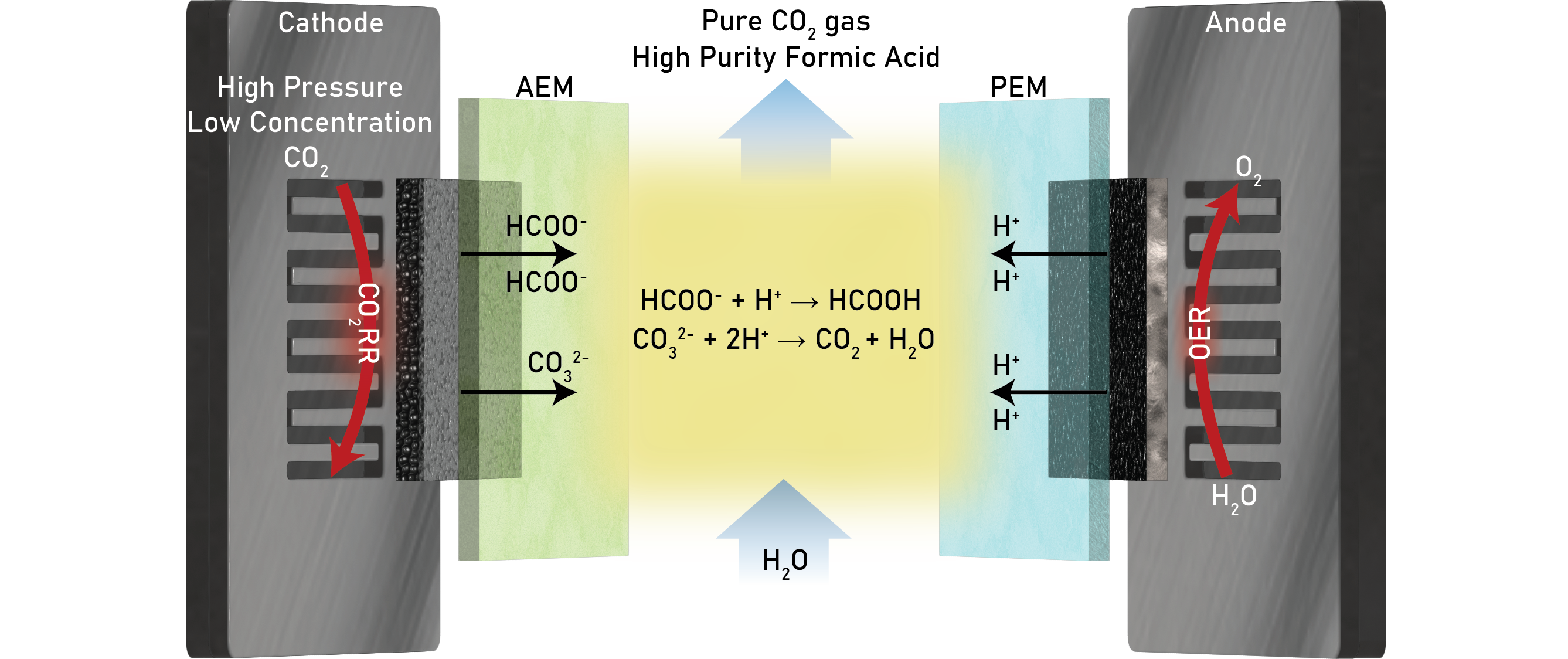
Electrochemical carbon dioxide reduction (CO2RR) to valuable fuels and chemicals has represented a promising carbon utilization technology. Highly selective catalysts and electrochemical devices have been demonstrated to efficiently reduce CO2 into formic acid but suffer kinetically when applied with dilute CO2 sources. Therefore, we present high-pressure CO2 (>1 atm) as a method to boost electrocatalytic activity and stability. High pressures can enhance CO2 coverage on the catalyst’s surface to convert dilute sources of CO2 (< 10 mol%), bypassing energy-intensive upstream CO2 purification. Here, we show that a stream of 10 mol% CO2 can achieve a 15.8% greater formic acid Faradaic Efficiency (FE, up to 91.2% total) at 100 mA cm-2 when pressurizing the gas stream to 5 atm. We also reach a formic acid FE of 81.0% utilizing 5 mol% CO2 at 10 atm, attaining a 33.2% FE improvement from 1 atm. With this enhancement, we demonstrate the stable performance of 5 mol% CO2RR at 20 atm and 50 mA cm-2 with >70% formic acid FE for up to 90 hours. Leveraging a porous solid electrolyte (PSE) reactor design with this high-pressure approach, we obtain a stream of high-purity formic acid. Simultaneously, we benefit from CO2 capture via the crossover of (bi)carbonate ions formed at the catalyst-membrane interface, allowing neutralization and release of high-purity CO2 gas from the middle layer. At 50 mA cm-2 and elevated pressures, we detected the crossover of carbonate and bicarbonate ions, rather than only carbonate species, improving the electron efficiency of carbon capture.