2025 AIChE Annual Meeting
(400s) High-Gradient Magnetic Separation and Phenotypic Characterization of Sickle Red Blood Cells
Authors
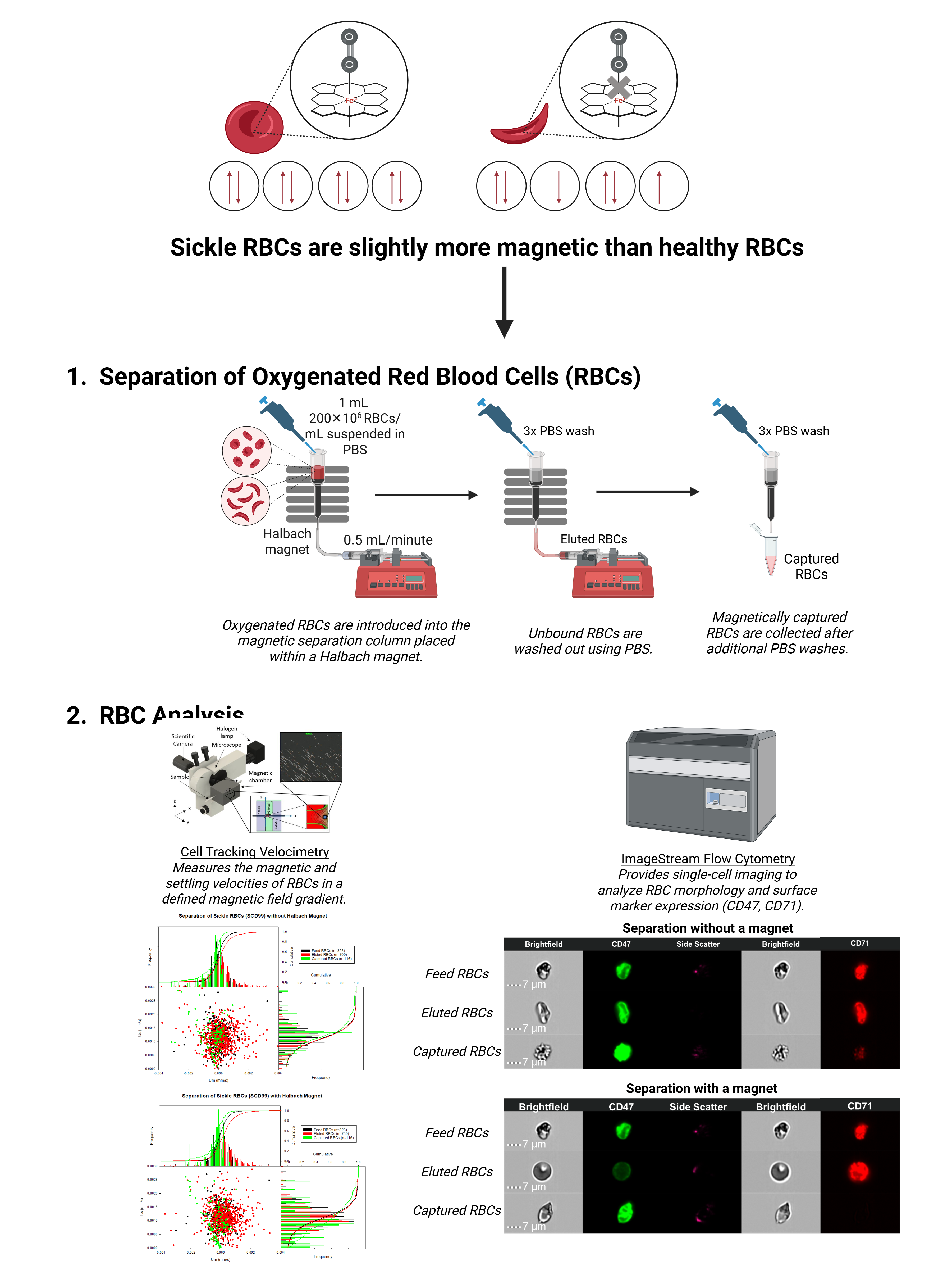
One potential strategy is the selective depletion of HbS RBCs based on their distinct magnetophoretic behavior, which is intrinsically linked to oxygen-binding affinity. The Fe²⁺ atom within each heme group of hemoglobin has an intrinsic magnetic moment of 5.46 Bohr magnetons. In its deoxygenated state, hemoglobin exhibits paramagnetic properties due to the presence of unpaired electrons. As oxygen binds covalently to Fe²⁺, the heme groups undergo a transition to a diamagnetic state. Since HbS RBCs exhibit a lower oxygen-binding affinity than healthy RBCs, their magnetic properties differ, enabling their selective separation using magnetic techniques. However, the difference in the magnetic susceptibility between sickle and healthy RBCs is relatively small, so high-gradient magnetic separation (HGMS) systems are employed to achieve effective separation.
HGMS relies on the generation of strong magnetic field gradients, typically achieved using columns packed with ferromagnetic material within an external magnetic field. These high gradients enable the capture of RBCs with subtle differences in magnetic susceptibility. The most widely used HGMS system is the magnetic-activated cell sorting (MACS) column developed by Miltenyi Biotech. These columns offer several advantages, including low cost, rapid sample processing, and compatibility with crude biological samples. These columns are widely used primarily due to their biologically invasive nature as there is no direct contact between the cells and the external magnets. They have been successfully employed in the separation of fetal and malarial red blood cells for both research and clinical applications. Additionally, there has been a shift in focus from labeled separations to label-free separation, eliminating the need for ligand-conjugated magnetic nanoparticles. This also removes the necessity of separating the captured cells from the nanoparticles via physical or chemical methods, thus preserving the native state of cells for downstream analysis. Therefore, label-free magnetic separation presents a non-invasive alternative for isolating healthy RBCs from sickle RBCs.
In this study, we conducted high-gradient magnetic separation of oxygenated RBCs from both healthy and SCD patients using a Miltenyi MiniMACS™ column combined with a Halbach array magnet. The separation was also performed without the external magnet as a control. To characterize the magnetic and phenotypic properties of the RBCs in the feed, eluted, and captured fractions, we employed two complementary techniques: cell tracking velocimetry (CTV) and imaging flow cytometry (IFC).
CTV integrates microfluidics and microscopy to quantify single-cell magnetic mobility under a well-defined magnetic field gradient. The CTV system used in this study consists of a borosilicate glass capillary positioned within a magnetic chamber, composed of N52 neodymium magnets and machined 1018 steel pole pieces. This setup allows for precise tracking of RBC trajectories under the combined influence of magnetic and gravitational forces. IFC, on the other hand, combines flow cytometry with fluorescence and optical microscopy to simultaneously assess RBC morphology and surface biomarker expression. Two key RBC biomarkers analyzed in this study are CD47 and CD71. CD47, also known as integrin-associated protein, plays a crucial role in regulating RBC clearance by macrophages. It is commonly referred to as a "don't eat me" signal, as its expression helps RBCs evade phagocytosis. Conversely, CD71, or the transferrin receptor, is highly expressed on reticulocytes—immature RBCs recently released from the bone marrow.
The results from CTV analysis revealed that the magnetic velocity distribution of the captured RBCs was centered around zero or in the negative region. This trend was observed in RBCs captured with and without the Halbach magnet. These findings suggest that under ambient oxygen conditions, RBC capture may be primarily driven by cell adhesion to the column packing material, rather than magnetic susceptibility alone. Additionally, a significant proportion of magnetic RBCs were found in the eluted fraction, indicating that the packing material did not generate a sufficiently strong magnetic field gradient to effectively separate sickle RBCs from healthy RBCs with only subtle differences in their magnetic susceptibilities.
IFC analysis provided further insights into the phenotypic characteristics of the separated RBC fractions. Notably, captured RBCs exhibited higher CD47 expression compared to feed and eluted RBCs. One possible explanation is that CD47 clustering within the captured fraction induces a conformational change, thereby acting as a “eat me” signal despite CD47's typical role in preventing macrophage-mediated clearance. This hypothesis is supported by the observation that captured RBCs also displayed lower CD71 expression, indicating a predominance of mature, possibly senescent RBCs within this fraction. Additionally, SCD patient samples exhibited significantly higher CD71 expression levels compared to healthy donor samples, consistent with the presence of a larger population of reticulocytes. This finding is indicative of increased RBC turnover, often observed in SCD patients.
One future research direction is the optimization of HGMS column design to improve the capture efficiency of magnetically distinct RBCs. To address this, future work will leverage computational modeling techniques such as COMSOL Multiphysics simulations and Abassov's magnetic filtration model to systematically evaluate the impact of packing material composition, column geometry, and applied magnetic field strength on separation performance. Additionally, further studies will investigate the role of RBC adhesion in the capture process by conducting adhesion assays to quantify RBC interactions with the column packing material. Understanding these adhesion mechanisms will be essential for refining separation protocols and minimizing non-specific cell retention.
In conclusion, this study demonstrates the feasibility of using high-gradient magnetic separation to selectively remove HbS RBCs from mixed RBC populations based on their differential magnetophoretic behavior. While current HGMS designs require further optimization to achieve clinically relevant separation efficiencies, our results provide valuable insights into the interplay between RBC magnetic properties, surface biomarker expression, and separation dynamics. By refining this approach, we aim to develop a scalable, antigen-independent transfusion strategy that reduces the burden of alloimmunization in SCD patients, ultimately improving transfusion safety and efficacy.