2025 AIChE Annual Meeting
A High-Efficiency Click Chemistry Ligation Strategy for Engineering of Antibody-Conjugated Lipid Nanoparticle Platforms
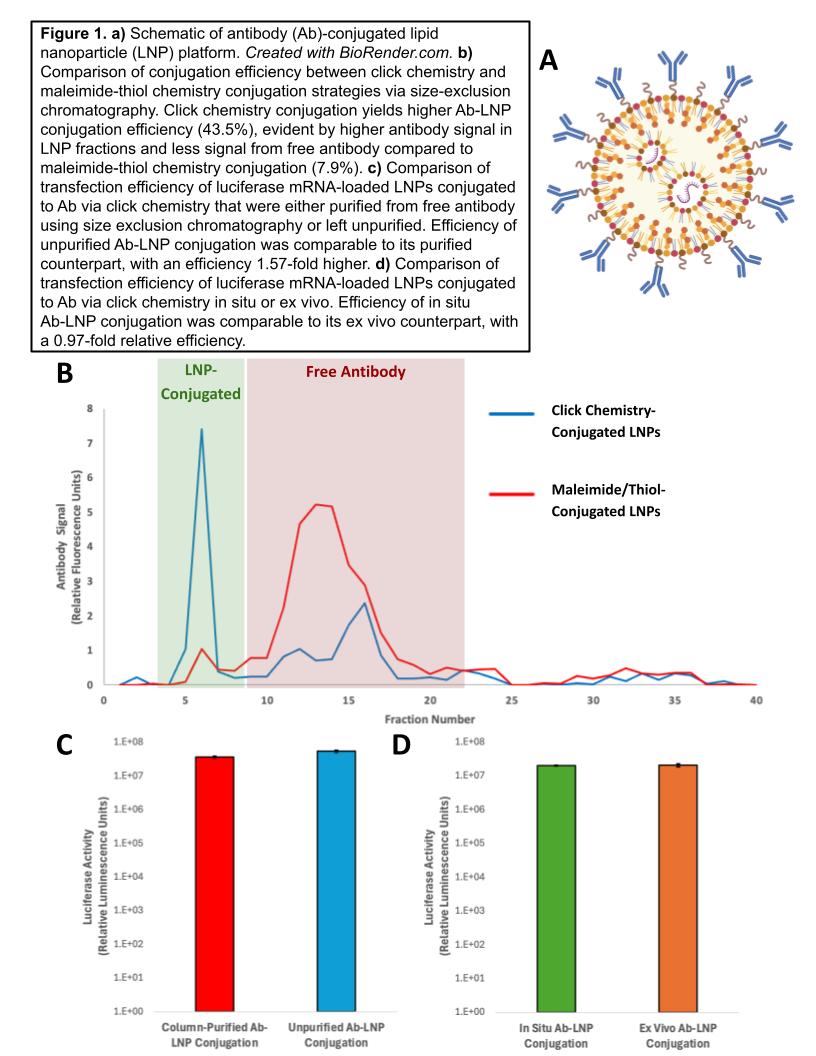
In this work, a novel click chemistry conjugation strategy is reported that addresses these drawbacks. This ligation reaction has the advantages of biorthogonality, rapid reaction kinetics, and improved conjugation efficiency, which is the amount of antibody bound to an LNP versus free antibody based on size-exclusion chromatography. Optimization of LNP formulations and conjugation conditions, namely ligation time and antibody concentration, with these new reagents saw conjugation efficiencies up to 93%.
The implications of this high reactivity are multiple. First, the high conjugation efficiencies afforded by this fast ligation reaction allow for comparable in vitro efficacy between column-purified and unpurified Ab-conjugated LNPs. Unpurified Ab-conjugated LNPs were 157% more effective at mRNA transfection of human umbilical blood-derived erythroid progenitor cells (HUDEP2 cell line) compared to column-purified Ab-conjugated LNP. This feature has the potential to facilitate rapid antibody and/or LNP formulation screening by bypassing time-consuming and loss-inducing purification steps. Additionally, the higher conjugation efficiencies afforded by this click chemistry ligation reduce excess antibody use while not denaturing the antibody. Second, the high reactivity of this novel strategy permitted successful in situ conjugation of antibody to LNPs in vitro. In situ targeting was 97% as effective as in vitro targeting in HUDEP cells using this approach. This in situ approach avoids the difficulties of reproducibility and reliability for ex vivo conjugation, posing advantages for downstream scalability of these Ab-LNP systems.
Overall, this work introduces an Ab-LNP click chemistry conjugation reaction that has significantly better conjugation efficiency due to faster reaction kinetics compared to currently established conjugation methods, expanding the therapeutic potential for Ab-conjugated LNP platforms.