2025 AIChE Annual Meeting
(72e) High Affinity Delivery of Nanobody-siRNA Conjugates Targeting Breast Cancer Oncogenes
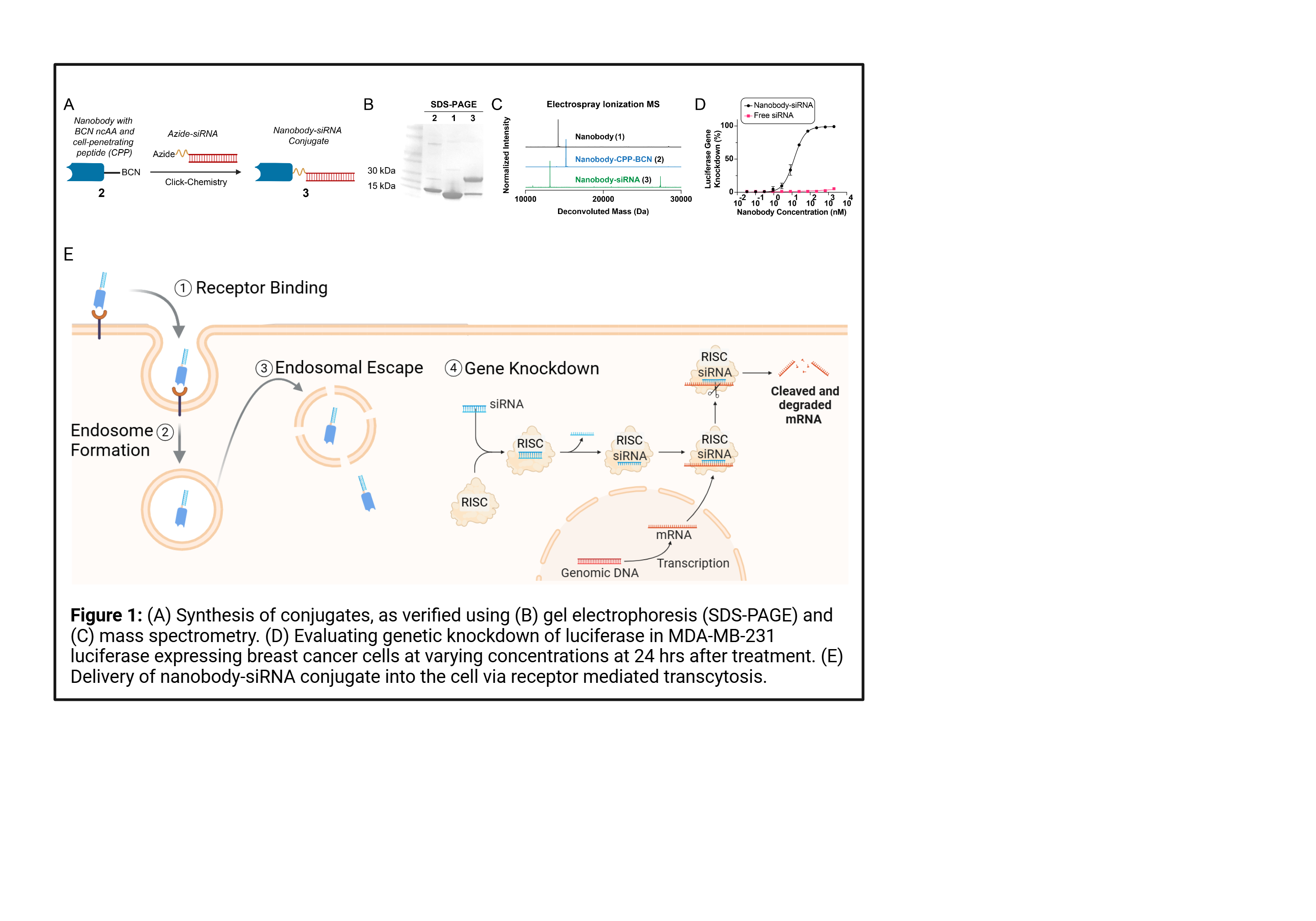
RNA therapy offers a logic-gated pathway to target genes of interest for cancer immunotherapy and autoimmune diseases. Of these nucleic acid therapies, short-interfering RNA (siRNA) has recently gained interest for both clinicians and engineers due to the ability of siRNA to decrease the expression of target genes, which can be selected to inhibit genes linked to the growth and development of cancer. Overexpression of oncogenes such as B-cell lymphoma 2 (BCL-2), polo-like kinase 1 (PLK-1), and N-Myc proto-oncogene protein (N-Myc) can lead to progression and metastasis of the tumor and are linked to progressive cases of breast cancer. Building upon our recently integrated strategies for the incorporation of noncanonical amino acids (NCAAs) into proteins in the Kimmel Lab, we engineered a nanobody-RNA conjugate using click chemistry to target cancerous tissues, delivering siRNA targeting these oncogenes into the tumor cells. This delivery platform relies on two elements: (1) high affinity binding to target receptors and (2) efficient siRNA delivery into the cytoplasm of the cancer cell. The addition of cell penetrating peptides (CPPs) into our design increases the amount of siRNA escaping encapsulation by endosomes, as seen through fluorescence microscopy of nPDL1-siLUC delivery to MDA MB 231 luciferase cells in vitro. High affinity delivery of nPDL1-siLUC and nAlb-siLUC was observed in vivo, ultimately knocking down luciferase production and verifying the performance of our modular delivery platform. We next looked at in vitro evaluation in EMT6 and 4T1 cancer models of our modular platform to demonstrate efficient gene knockdown of target oncogenes at mRNA and protein levels, while maintaining low cytotoxicity, confirmed via both qPCR and ELISA. We then investigated the immunogenetic impact of targeting these genes in vivo through systemic delivery in pharmacokinetics, biodistribution, and toxicity studies. Our modular platform is shown to enhance the delivery of siRNA payloads in vitro and in vivo to targeted cells invoking an immunogenetic response to decrease tumor growth. Further, our nanobody-RNA conjugate design strategy will allow us to explore the interaction between immune pathways and gene modification in cancer and autoimmune diseases to provide novel targets for immunotherapies.