2025 AIChE Annual Meeting
(385bd) Herbicides of Nonanoic Acid from Oleaginous Microorganisms (Rhodotorula glutinis and Yarrowia lipolytica) Via in-Situ Ozonolysis of Accumulated Oils
Authors
The sustainable production of nonanoic acid, a medium-chain fatty acid, indicates an advancement in renewable chemical manufacturing, reducing environmental impact and lowering carbon emissions. Nonanoic acid is an environmentally friendly herbicide that controls weeds in food crops, golf courses, and greenhouses. Moreover, it makes blossoms thinner for fruit trees to improve yield quality [1]. Herbicides that contain nonanoic acid offer several benefits compared to traditional herbicides, as nonanoic acid acts as a contact herbicide, rapidly breaking down the protective leaf cuticle to weeds, leading to desiccation and visible damage within minutes to hours [2]. Additionally, when combined with glyphosate, nonanoic acid enhances the uptake and performance of glyphosate, providing both rapid burndown and long-term weed control [3].
The global nonanoic acid market projects to grow at a compound annual growth rate (CAGR) of approximately 6% over the next five years [4]. The increasing demand for sustainable and eco-friendly chemicals across various industries, including personal care, agriculture, and food processing, fuels this growth. Nonanoic acid is widely used as an emulsifier and skin-conditioning agent in cosmetics and skin care products, driven by consumer preference for natural and biodegradable ingredients[5].
Nonanoic acid produced from microorganisms is more sustainable than traditional chemical synthesis methods. The microbes utilize renewable feedstocks, such as glucose, glycerol, and volatile fatty acids, as their carbon sources. This approach reduces the dependence on petroleum-derived raw materials and aligns with green chemistry principles. In addition, implementing situ ozone cracking skips the lipid extraction step, reducing the process complexity, operational costs, and environmental footprint. Moreover, the oleaginous yeast can grow on industrial waste streams, converting them into valuable products, providing a circular economy advantage, and turning waste into bio-based chemicals [6]
This study investigates the application of in situ ozone cracking to directly convert lipids from oleaginous yeasts, Rhodotorula glutinis, and Yarrowia lipolytica to produce valuable nonanoic acid. Research finding indicates that R.glutinis accumulates lipid with more than 61% of oleic acid when cultured in optimized media ( glucose rich conditions), meeting biodiesels standards [7]. A high C/N ratio triggers nitrogen depletion, redirecting metabolic flux from protein synthesis to lipid storage. The surplus carbon supply provides acetyl-CoA, the building block of fatty acids. At the same time, the scarcity of nitrogen limits the protein and nucleic acid synthesis, leading to less cell division and redirecting resources toward lipid storage [8].
Lipid production in oleaginous microorganisms starts at the end of the exponential phase and becomes more intense during the stationary phase. Under nitrogen-limiting conditions and an excess of carbon, the yeast cells will initially grow, consuming both nutrients. However, as the nitrogen source is depleted, cell proliferation slows down, and the yeast enters the stationary phase. There are numerous factors affecting the lipid content and fatty acid composition, e.g., environmental factors including aeration, pH, temperature, inoculum size, incubation period, medium composition, and culture conditions. However, the most important factor is the microorganism used for lipid production[3]
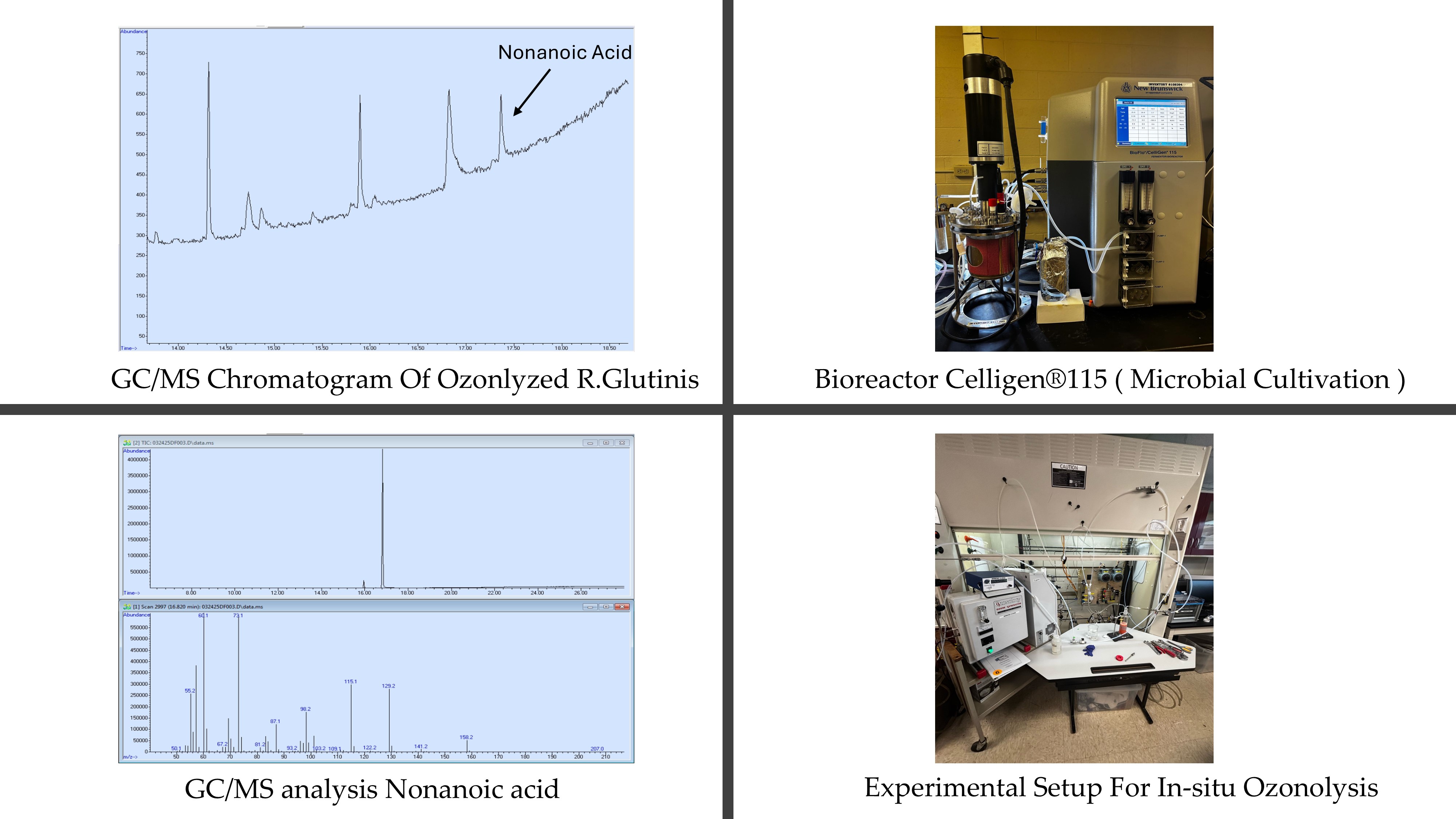
The yeasts ( Rhodotorula glutinis and Yarrowia lipolytica) were pre-grown in YM medium ( 3 g.L-1 yeast extract, 3 g.L-1 malt extract, 5 g.L-1 peptone, 10 g.L-1 glucose ) and were preserved in cryo-stocks ( 500 μL of yeast culture, 200 μL pure glycerol). The yeast cultivation was carried out in 900 ml fed-batch bioreactor CelliGen®115 ( New Brunswick Scientific Co., INC., Edison, NJ, USA) and baffled Erlenmeyer flasks filled with 200 ml of the medium. The temperature was kept at 25°C, the recommended growing condition from ATCC, and the pH of the media was initially set to 6.2.
The growth curve of Rhodotorula glutinis and Yarrowia lipolytica exhibits an initial lag phase of approximately 25 hours with biomass production peaking at around 72 hours for Rhodotorula glutinis and 75 hours for Yarrowia lipolytica. Lipid production begins at the exponential growth phase, intensifying during the stationary phase, which was identified as the optimal timing for ozonlysis. The growth performance of the yeasts Rhodotorula glutinis and Yarrowia lipolytica varied notably under different carbon-to-nitrogen (C:N) ratios and feeding strategies. Initially, R. glutinis grew best at a low C:N ratio (8), reaching peak biomass within 100 hours, whereas higher C:N ratios led to lower biomass and longer growth times. Similarly, Y. lipolytica thrived best at a lower C:N ratio (8), achieving its highest biomass within 200 hours, with significantly reduced biomass at higher ratios (100). Interestingly, when glucose feeding was extended at a moderate C:N ratio (70) with a prolonged exponential growth phase, both yeasts demonstrated substantially enhanced growth, with R. glutinis slightly outperforming Y. lipolytica in biomass yield and achieving peak growth faster (75 vs. 72 hours). Under optimized fed-batch bioreactor conditions, both yeasts maintained strong growth, again with R. glutinis marginally surpassing Y. lipolytica in biomass accumulation, indicating its potential advantage in controlled, nutrient-rich environments.
The in situ ozone treatment was initiated precisely during the stationary phase of yeast growth. This ozone treatment effectively converted membrane lipids from oleaginous yeasts—predominantly oleic acid—into nonanoic acid without requiring preliminary lipid extraction. The average ozonolysis duration was approximately 30–35 minutes for Rhodotorula glutinis, whereas Yarrowia lipolytica required significantly less time, around 8–12 minutes. After completion of the ozonolysis, the solution was allowed to rest for 24 hours, facilitating the formation of two distinct layers: an aqueous phase and a liquid phase. Nonanoic acid was observed predominantly in the aqueous phase.
Chloroform extraction was employed to recover the nonanoic acid from the aqueous phase of Yarrowia lipolytica and Rhodotorula glutinis ozonized sample, and the purity of the extracted acid was confirmed using gas chromatography-mass spectrometry (GC-MS), evidenced by a clear GC peak at 16.826 minutes and characteristic mass spectral fragments at m/z 73, 115, and 129 for Yarrowia lipolytica. The gas chromatography analysis for Rhodotorula glutinis revealed a distinct peak corresponding to nonanoic acid, with a retention time of approximately 16.82 minutes. The mass spectrum of this peak displayed characteristics of fragment ion at m/z values of 60.1, 73.1, 115.1, 129.2, and a clear molecular ion peak at 158.2, which matched closely with reference spectra from the NIST mass spectral library. The library search yielded a high match factor of 918, confirming the reliable identification of nonanoic acid.
This method streamlines the production process and reduces costs by eliminating energy-intensive lipid extraction steps while aligning with sustainable biomanufacturing principles through renewable feedstocks and a more efficient conversion process. The findings highlight the potential of microbial lipid platforms for sustainable industrial chemical synthesis, addressing current industry demand for sustainability and actively contributing to global efforts toward greener industrial practices.
References
[1] “NONANOIC ACID,” Ataman Kimya. Accessed: Apr. 07, 2025. [Online]. Available: https://www.atamanchemicals.com/nonanoic-acid_u31000/
[2] admin, “Does that new, fast acting, ready-to-use, garden herbicide really work?,” Eureka! Accessed: Apr. 07, 2025. [Online]. Available: https://eurekaag.com.au/dortusreallywork/
[3] “NONANOIC ACID,” Ataman Kimya. Accessed: Apr. 07, 2025. [Online]. Available: https://www.atamanchemicals.com/nonanoic-acid_u31000/
[4] M. R. Intellect, “Navigating Growth- Key Drivers in the Nonanoic Acid Market,” Market Research Intellect. Accessed: Apr. 07, 2025. [Online]. Available: https://www.marketresearchintellect.com/blog/navigating-growth-key-driv…
[5] “Pelargonic Acid (Nonanoic Acid) | Food Grade & Biodegradable,” Consolidated Chemical. Accessed: Apr. 07, 2025. [Online]. Available: https://consolidated-chemical.com/product/pelargonic-acid/
[6] S. Donzella et al., “Recycling industrial food wastes for lipid production by oleaginous yeasts Rhodosporidiobolus azoricus and Cutaneotrichosporon oleaginosum,” Biotechnol. Biofuels Bioprod., vol. 15, p. 51, May 2022, doi: 10.1186/s13068-022-02149-3.
[7] D. D. Maza, S. C. Viñarta, Y. Su, J. M. Guillamón, and M. J. Aybar, “Growth and lipid production of Rhodotorula glutinis R4, in comparison to other oleaginous yeasts,” J. Biotechnol., vol. 310, pp. 21–31, Feb. 2020, doi: 10.1016/j.jbiotec.2020.01.012.
[8] K. M. Palanisamy et al., “Lipid Enhancement in Oleaginous Nannochloropsis sp. under Nitrate Limitation for Future Bioenergy Production,” Int. J. Energy Res., vol. 2023, pp. 1–8, Dec. 2023, doi: 10.1155/2023/5412660.