2025 AIChE Annual Meeting
(385i) Hemocompatibility Evaluation Via Fluid Mechanics and Erythrocyte Derived Extracellular Vesicle Production
I am a Ph.D. candidate in Dr. Edgar O’Rear’s Biorheology Lab at the University of Oklahoma, with plans to defend this academic year. My dissertation research primarily focuses on hemocompatibility of blood contacting devices. Specifically I evaluate damage to red blood cells(RBC), primarily using the release of extracellular vesicles(EV) as a damage marker, post exposure to non-physiologic forces often experienced in mechanical circulatory support(MCS) devices. I have several years of industry experience at a medical device startup(VADovations Inc), working with Dr. Trevor Snyder developing a ventricular assist device(heart pump), which sparked my research interests and motivated me to pursue my doctorate.
Research Interests:
Highlights: Hemocompatibility, Fluid Mechanics(medical device design and testing, microfluidics, bioreactors, biorheology/rheology), Flow Cytometry panel design, Biomarkers for diagnostics and predictive use from a variety of cell types, Extracellular Vesicle characterization and phenotyping.
My main research interests and expertise are in hemocompatibility of blood contacting devices, primarily regarding the fluid mechanics of said devices and monitoring the effects of the fluid mechanics(i.e. non-physiological levels of fluid stress) on blood components to identify potential diagnostic and predictive biomarkers, primarily through monitoring EV release. I have a solid understanding and foundation in fluid mechanics and am interested in applying that knowledge across a wide variety of fields including medical device development, microfluidic design/fabrication, and bioreactor improvement, among others. I’m also interested in further exploring the effects of fluid stresses on a variety of cell types by designing and modeling microfluidic devices as well as employing other rheological methods and monitoring the effects through flow cytometric panel design and other relevant assays.
I have extensive laboratory experience and am adept in the following skills/techniques: flow cytometry and panel design/development and analysis(several years of experience), rheometry, nanoparticle tracking analysis, microscopy(light and SEM), python, R, mathematical modeling and design/fabrication of microfluidic devices, statistics and data analysis, and a variety of biologic assays(e.g. hemoglobin, lactase dehydrogenase, thrombin generation). I have supervised and trained undergraduate and other graduate students in their research using many of these methods.
Introduction:
MCS is a lifesaving technology that uses implanted or extracorporeal pumps to assist in circulating patient’s blood for those experiencing compromised cardiopulmonary function. Unfortunately, MCS patients still experience complications and adverse events often attributed to device hemocompatibility issues deriving from non-physiologic fluid stresses imposed by the pumps. Historically, MCS development has focused on minimizing hemolysis through the minimization of shear imposed by devices. While this approach has improved MCS treatment, adverse events, such as thrombosis and bleeding, persist. It is widely accepted that other markers of blood damage, aside from hemolysis, need to be better understood and evaluated in MCS devices to further understand remaining hemocompatibility issues.
We are primarily interested in evaluating the utility of erythrocyte derived EVs(ErEV) as a potential damage marker during benchtop device testing as well as their potential use as a diagnostic tool in MCS patients. Using ErEVs as a damage marker is of interest for a variety of reasons. First, MCS patients have elevated levels of EVs, including ErEVs, associated with adverse events, and it has been shown that EV concentrations correlate with pump flow rate. ErEVs also have a direct tie to thrombotic events due to their prothrombotic nature deriving from an anionic surface charge via the externalization of phosphatidylserine(PS). Additionally, ErEVs may serve as a harbinger of adverse events. Two projects, described below, were undertaken to evaluate and better understand the conditions that generate ErEVs.
Project 1-Sensitivity of RBCs to Mechanical Trauma: ErEVs versus Hemolysis
We have been investigating the sensitivity of RBCs to mechanical trauma as gauged by EV production. Hemolysis is commonly used to evaluate hemocompatibility of devices during benchtop testing, with current FDA approved devices causing minimal amounts of hemolysis, yet adverse events persist. Identification of a more sensitive marker to mechanical trauma is paramount to reveal remaining hemocompatibility issues and further improve device design. We tested porcine and bovine RBCs as these species are often utilized for in vitro device evaluation as surrogates for human. We hypothesize that ErEVs will be a more sensitive marker of mechanical trauma than hemolysis in all species tested, providing earlier insight into potential hemocompatibility problems that hemolysis alone would not identify.
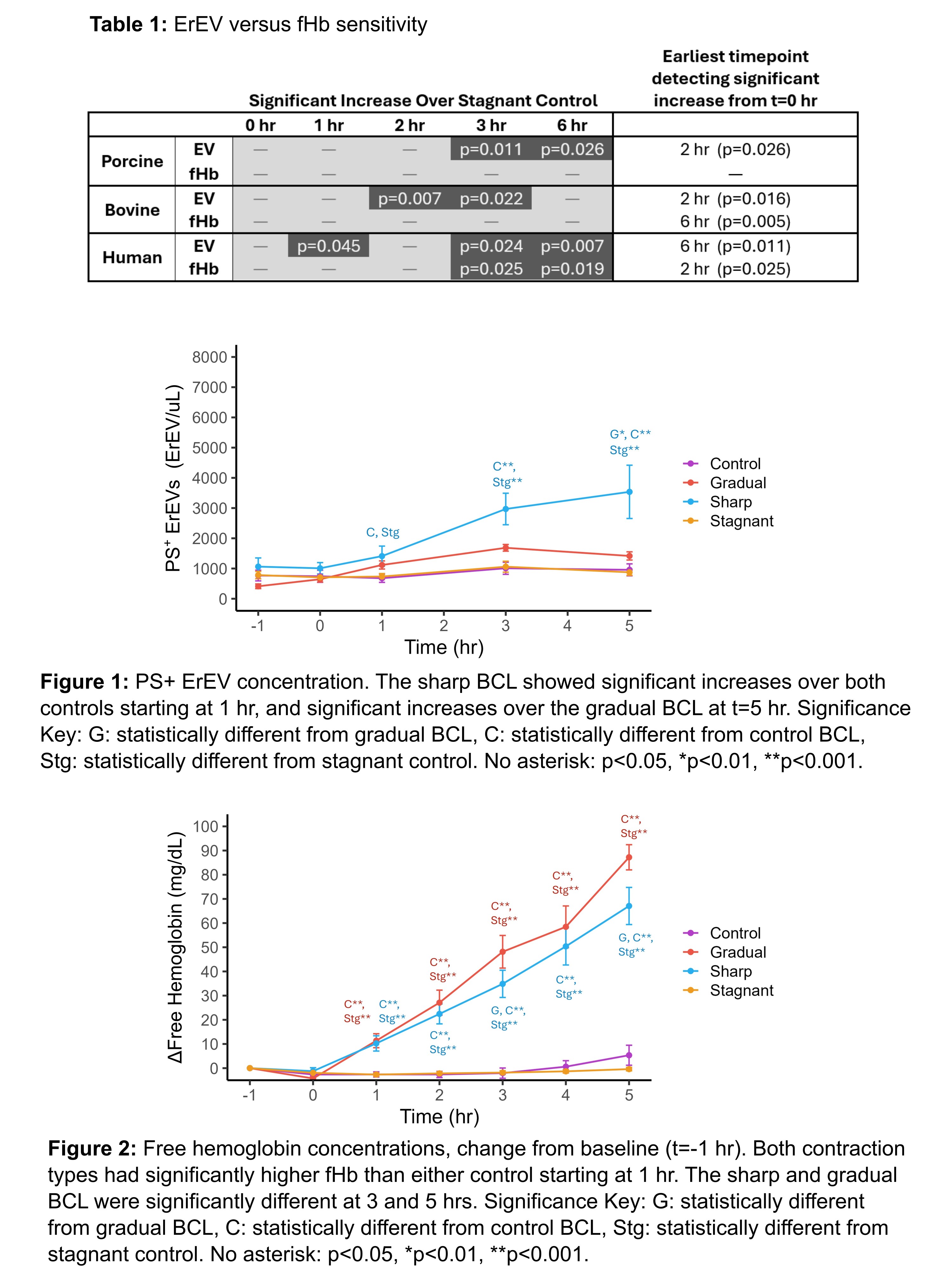
Methods: This hypothesis was tested by perfusing isolated RBCs(porcine, bovine, human) through benchtop circulatory loops(BCL) over 6 hrs, a common setup for evaluating MCS pumps. The CentriMag blood pump was used at clinical operating conditions. Hemolysis was monitored(Harboe assay) through free hemoglobin(fHb) concentrations as well as through the normalized index of hemolysis(NIH). ErEVs were measured using flow cytometry by staining for species dependent parent cell markers and PS externalization. Sensitivity was examined through statistical testing by identifying the earliest timepoints significant differences were detected from the start of perfusion(one-way repeated measures ANOVA with post-hoc testing) or respective stagnant controls(Welch’s t-test). Data is presented as mean±SEM.
Results and Discussion: For all species, 6 hours of perfusion resulted in ErEV increases of 1,509±263, 2,289±534, and 10,830±2,755 ErEV/μL over baseline values(i.e. bulk sample) for human, porcine, and bovine, respectively. Increases in fHb over baseline were 8.3±1.5, -0.54±0.95 and 49.3±19.6 mg/dL, respectively. NIH values were similar or less than expected values for the CentriMag at comparable conditions(~0.001g/100L) for all species. No discernable changes in ErEVs or hemolysis for any stagnant controls were detected. Table 1 summarizes the timepoints with p-values where significant changes were detected(with time or over stagnant controls) for both ErEV and fHb. ErEVs are more sensitive(earlier detection of significance) for perfusion of both porcine and bovine RBCs, while results are mixed for human. NIH values show that all levels of hemolysis would be regarded as insignificant(including human), despite having detectable ErEV levels. Despite the mixed results for human in Table 1, NIH indicates ErEVs are likely more sensitive for human as well.
Conclusion: ErEVs are a more sensitive damage marker in bovine and porcine models. ErEVs could be used in MCS testing to indicate issues that hemolysis would not detect.
Project 2-Extensional Stress Generates More ErEVs than Shear
After establishing that ErEVs are a more sensitive marker of damage especially in porcine and bovine models, we next wanted to determine which fluid stress types might be more damaging. In MCS development, extensional stresses are often neglected due to less pervasive presence in devices compared to shear. Recent work indicates extensional stresses may be a larger factor than previously considered as they cause higher levels of cellular deformation than shear of equal magnitude. This agrees with GI Taylor’s fundamental work on liquid droplet deformation and emulsion formation. As such, we sought to examine the effect of extensional and shear stress on ErEV release. We hypothesize that extensional stresses cause increased ErEV generation compared to shear. This work not only has application to MCS device development, but blood contacting devices in general(canula, prosthetic heart valves, etc.) and applications in bioreactors and biopharmaceutical engineering, and anywhere where the effect of fluid stresses on cells are relevant.
Methods: To examine the effect of shear versus extensional stress, isolated porcine RBCs were perfused for 5 hrs at 1.5 L/min through a BCL similar to project 1 with the addition of a sharp(3:1) or gradual contraction into the flow field. Extensional stress dominates in the sharp contraction while shear dominates in the gradual, allowing us to probe the effect of each stress type. This setup was inspired by the FDA critical path initiative nozzle, designed to emulate MCS environments, which uses similar contractions. Two controls were utilized: a BCL without a contraction and a stagnant control. Similar to project 1, ErEVs and hemolysis were measured and one-way ANOVAs performed.
Results and Discussion: ErEV concentrations increased for both contraction types and remained constant for both controls(Figure 1). Sharp BCL ErEV concentrations were significantly higher than controls starting at 1 hr(p<0.05) and were consistently higher than the gradual BCL but only significantly higher at 5 hours(p<0.01). Hemolysis trends were similar(Figure 2). Sharp and gradual BCL fHb levels were significantly higher than controls beginning at 1 hour (p<0.001), and significantly different from each other at 3 and 5 hours only(p<0.05), while NIH values between contraction types were not significantly different. While both ErEVs and fHb increased with perfusion through either contraction, the difference in hemolysis(fHb and NIH) was minimal, while the sharp BCL produced significantly more ErEVs compared to the gradual BCL. This supports our conclusion from project 1 and shows that extensional stresses are more damaging to RBCs than shear stresses as measured by ErEV production, at this condition.
Conclusion: More work is needed at a variety of flow rates to assess the differences in damage from extensional or shear stresses, but this study indicates that extensional stresses are more damaging. Again with potentially far reaching applications to MCS, biopharma, tissue engineering, and bioreactors in general.