2025 AIChE Annual Meeting
(178j) Hematocrit Separation Using Continuum-Based Diffusive Flux Model in a Trifurcated Microchannel
Authors
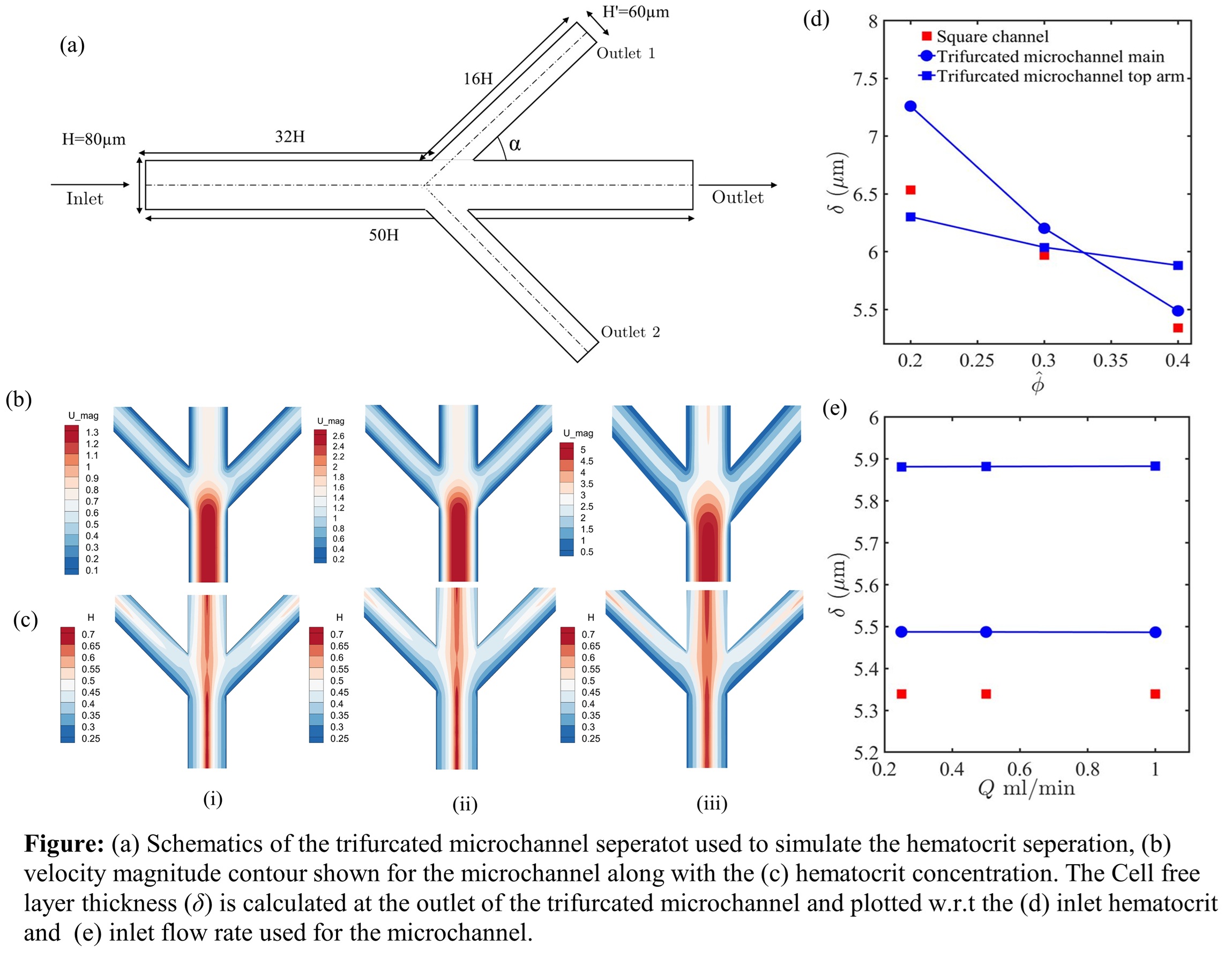
The particulate nature of blood, specifically the role of red blood cells and their influence on flow distribution, becomes noticeable when the channel cross-section is comparable to the length scales of RBCs. Platelets are significantly smaller in size and number density and are less significant from a rheological perspective. Fahreuss observed a distinctive behaviour of RBCs in blood flow within microchannels, with a migration towards the centre of the vessel of diameter less than 300 micron. Acrivos observed a similar phenomenon in suspension experiments. These observations were mathematically explained by Philips, who delineated the fluxes of particle migration and collision frequency during species transport. More realistic blood flow studies can be conducted by integrating the Philips formulation within the continuum approach, coupled with realistic viscosity models. A detailed viscosity model developed by Apostolidis and Beris for blood is incorporated in the present work to explore its rheological and particulate aspects. The primary objective of the present study is to optimise microfluidic design parameters for achieving significant hematocrit separation. A comprehensive examination of geometrical parameters is conducted within a trifurcated channel, first described in an earlier study.
The primary objective of this study is to analyse and optimise hematocrit separation using a trifurcated microchannel. The research focuses on understanding how geometric parameters, flow conditions, and hematocrit levels influence RBC separation efficiency and the fluxes responsible for shear-induced migration. This study uses computational fluid dynamics (CFD) simulations to provide insights into the governing mechanisms and optimal design parameters for effective hematocrit separation. A single-phase treatment of blood is adopted along with shear-induced particle migration for the red blood cells. The hematocrit distribution in the channel is determined by solving a diffusive flux model (DFM) equation jointly with the mass and momentum equations. The effective viscosity of blood is determined locally by using the Beris model. Simulations clearly show an improvement in separation efficiency for narrower gaps and dilute hematocrit concentration. Efficiency is only marginally affected by the angles of the separator arms and flow rates. These results help design a viable RBC-platelet separation device for clinical diagnostics.