2025 AIChE Annual Meeting
(173e) Halide-Driven Electrochemical CO? Conversion: Lower Cell Potentials and Enhanced C?? Product Selectivity
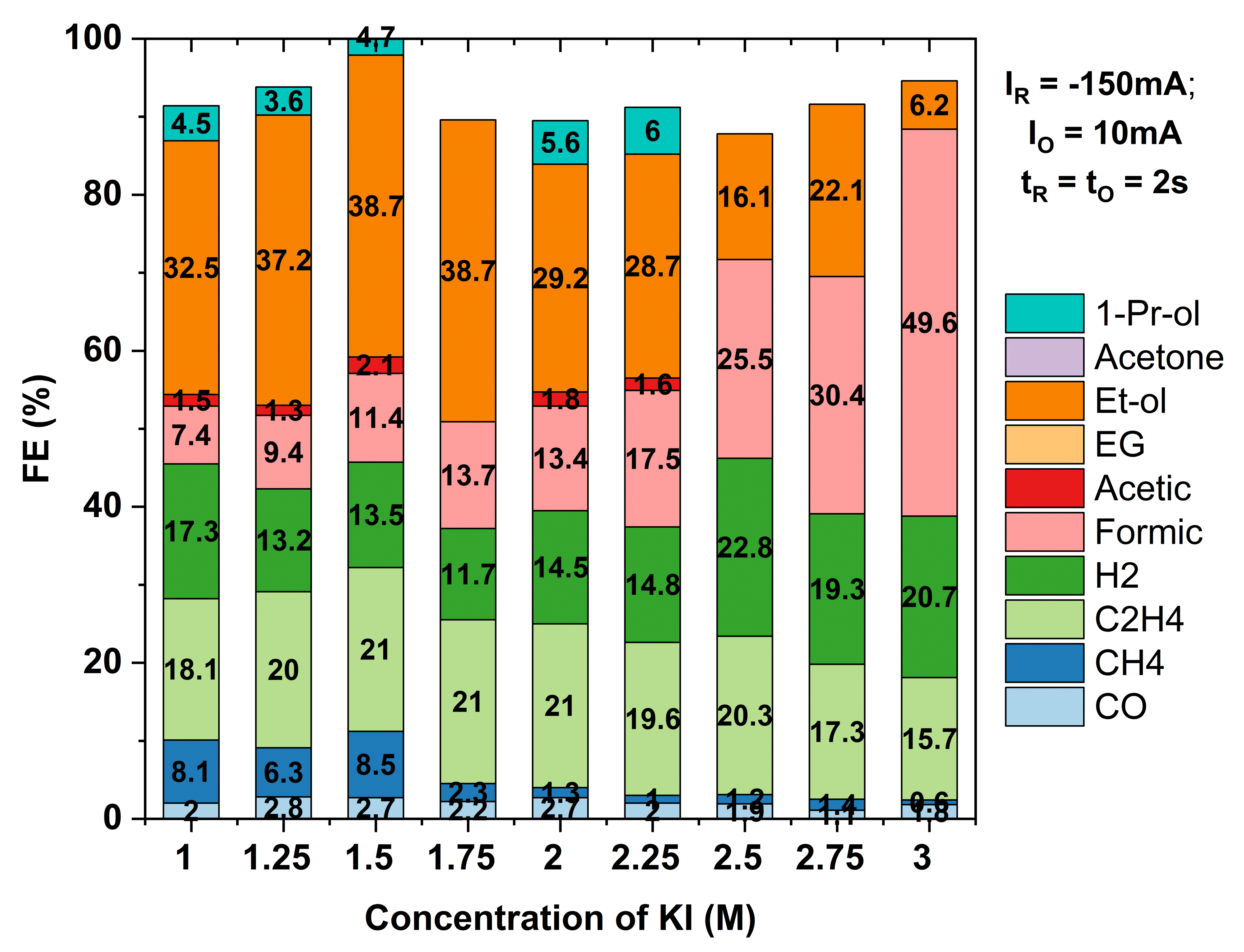
The electrochemical reduction of CO₂ to multicarbon (C₂⁺) products offers a sustainable pathway to renewable fuels and chemicals but remains challenged by high energy demands and limited selectivity. In this work, we demonstrate that the strategic incorporation of halide ions (Cl⁻, Br⁻, I⁻) into the electrolyte plays a dual role—lowering the cell potential and enhancing selectivity toward C₂⁺ products, particularly ethylene and ethanol. Halide ions influence CO₂ reduction by modulating the catalyst surface structure, stabilizing Cu⁺ (Cu(I)) oxidation states, and promoting C–C coupling pathways. Their high ionic conductivity also contributes to a reduction in overall cell potential. To further enhance performance, we couple halide-mediated surface engineering with a pulsed electrolysis strategy, consisting of alternating reduction and oxidation currents that dynamically tailor the local microenvironment. The pulsed electrolysis consists of six-segment waveform which is designed first to stabilize the Cu⁺ state, followed by its reduction, generating localized alkaline conditions conducive to C–C coupling. Optimization of halide concentration (0.1 M–3 M) and cathodic current density (50–300 mA/cm²) reveals iodide ions (I⁻) as the most effective, achieving the highest Faradaic efficiencies (FEs) and lowest cell potentials. Using in situ characterization techniques and high-throughput screening, we demonstrate that this combined halide–pulse electrolysis approach significantly reduces energy input while maintaining high C₂⁺ selectivity at commercially relevant conditions (≥150 mA/cm²). This synergy leads to FEs of 75% for C₂⁺ products, 60% for total liquid products, and 39% for ethanol at an applied current density of 150 mA/cm² and a cathodic potential of –1.1 V vs. RHE. These insights advance the rational design of efficient and scalable electrochemical platforms aligned with the U.S. Department of Energy’s mission for clean fuels and carbon-negative technologies.