2025 AIChE Annual Meeting
(130h) From Grotthuss Transfer to Conductivity: Machine Learning Molecular Dynamics of Aqueous KOH
Authors
Recent advances in molecular simulation methods allow for parametrizing molecular force fields from ab initio data using machine learning. We applied this technique to overcome the aforementioned limitations of classical and ab initio molecular dynamics. Over 50 000 Grotthuss transfer events in aqueous potassium hydroxide are sampled with our machine learning molecular dynamics approach. Simulations of all relevant hydrogen isotopes (hydrogen, deuterium, and tritium) are performed, and an in-depth statistical analysis of these reactions confirmed that the local structure around hydroxide ions is key to understand Grotthuss transfer. For example, our work showed that the hydroxide loses a hydrogen bond just before Grotthuss transfer takes place for all investigated isotopes. This quantifiably confirmed the qualitative finding by Tuckerman et al. [1, 2] from a very different approach.
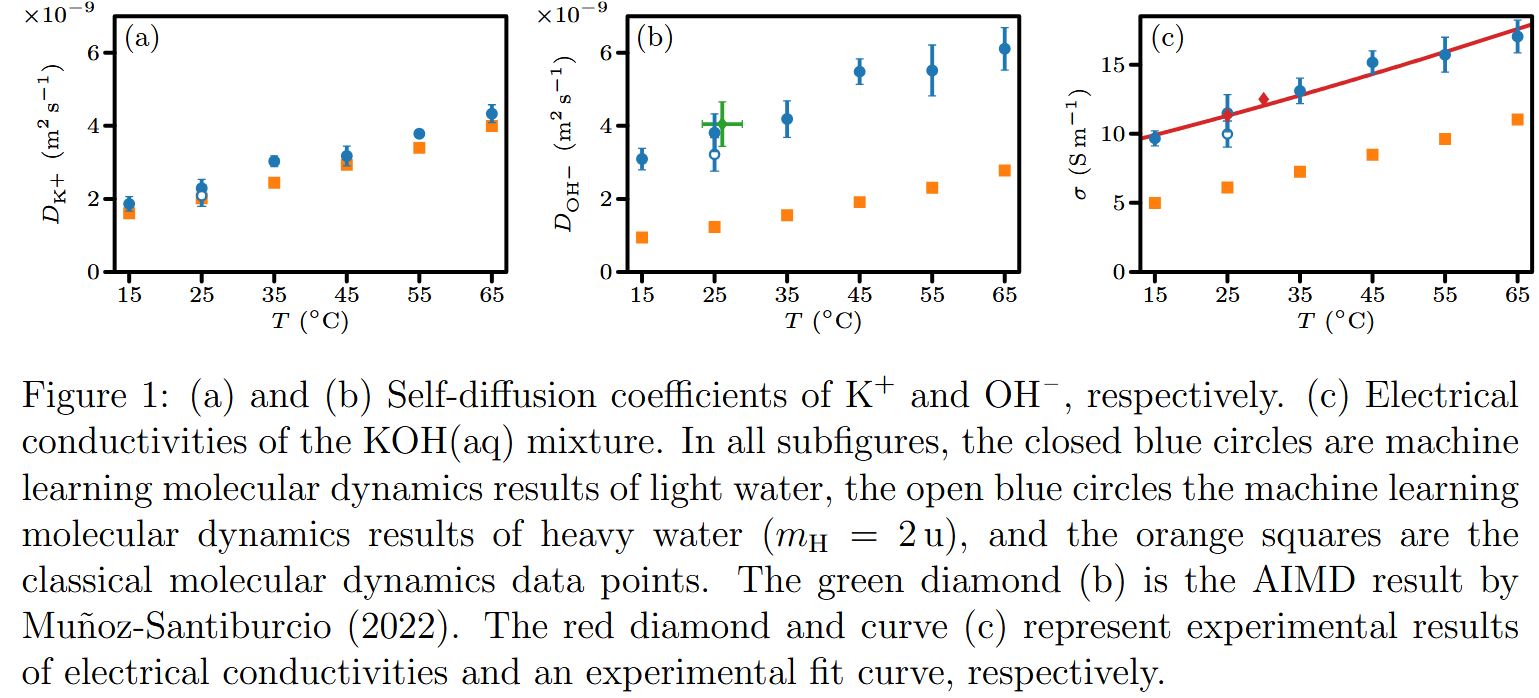
Reaction rates are computed at multiple temperatures from which the reaction barrier for Grotthuss transfer of hydroxide is determined. The reaction barrier is very similar to the energy required for breaking hydrogen bonds. This, and the loss of a hydrogen bond just before the reaction suggests that the rate-limiting step of Grotthuss transfer is the breaking of the hydrogen bond itself. This indicates that the transfer of the hydrogen atom between the water and hydroxide molecules is not the primary bottleneck in the reaction. Our simulations were long enough to compute self-diffusion coefficients of hydroxide and potassium ions, as well as electrical conductivities are quantitatively determined for multiple state points for the first time (Fig. 1.). Our simulations can now accurately reproduce experimental electrical conductivities of aqueous potassium hydroxide. This is a breakthrough in the field of chemical physics, as this is only possible by performing long simulations with an ML force field which captures Grotthuss transfer.
Currently, we are using these findings to improve potassium hydroxide mixtures for electrolyzers, where higher electrical conductivity reduces resistivity losses, therefore increasing overall electrolyzer efficiency. Our results suggest that weaker hydrogen bonding reduces the energy barrier for Grotthuss transfer. Therefore, we are mixing cheotropes, known for weakening hydrogen bonds, into our aqueous potassium hydroxide mixtures. This adds chemical complexity to the system, more chemical species at different concentrations, which creates new challenges for accurate ML force fields.
[1] M. E. Tuckerman, D. Marx, and M. Parrinello, “The nature and transport mechanism of hydrated hydroxide ions in aqueous solution,” Nature, vol. 417, no. 6892, pp. 925–929, Jun. 2002, doi: 10.1038/nature00797.
[2] M. E. Tuckerman, A. Chandra, and D. Marx, “Structure and Dynamics of OH-(aq),” Acc. Chem. Res., vol. 39, no. 2, pp. 151–158, Feb. 2006, doi: 10.1021/ar040207n.