2025 AIChE Annual Meeting
(553a) Glassy Gels Via Ionic Liquid Crosslinking
Author
Michael D. Dickey - Presenter, North Carolina State University
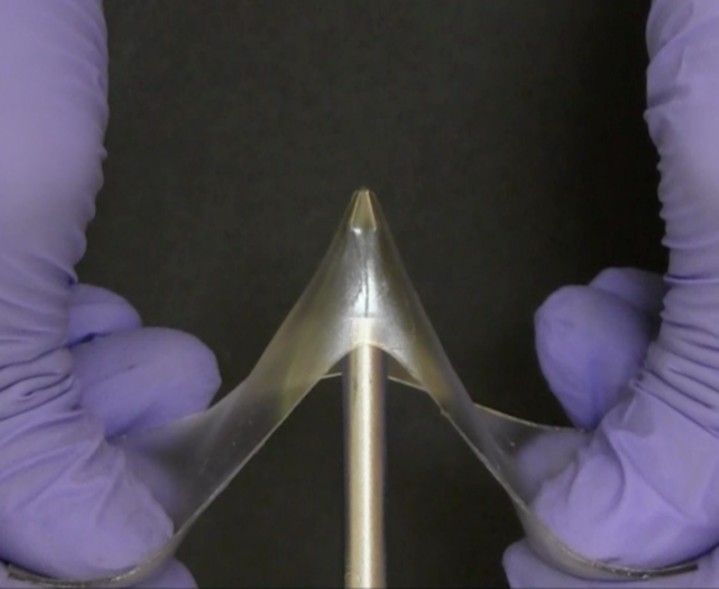
Glasses and gels are usually considered distinct classes of polymeric materials. Glassy polymers are generally hard (high modulus), possess a glass transition temperature, and are often brittle. Gels are polymer networks swollen with liquid. Usually, the presence of solvent in a crosslinked polymer network causes it to swell, thereby significantly decreasing the modulus and significantly increasing the strain at break. We discovered a class of materials that we call “glassy gels” that contain up to 60% liquid, yet have glassy mechanical properties [Nature, 2024]. The solvent in this case is an ionic liquid, which acts as noncovalent crosslinker between chains, restricting the movement of the polymer chains, thereby making the gel glassy at room temperature, resulting in an ultrahigh modulus (~1 GPa) and exceptional toughness, similar to thermoplastics. Yet unlike thermoplastics, which typically form using specialized catalysts and elevated temperatures, these “glassy gels” can form easily by mixing the ingredients and curing it with light via free radical polymerization. In addition, the glassy gels have useful properties such as self-healing, shape memory, and exceptionally strong adhesion to many surfaces despite being glassy. The findings should expand the applications of ionogels, which are compelling materials for energy storage devices (batteries), ionotronics (electrical devices that use ions instead of electrons), and actuators due to their excellent ionic conductivity, thermal and electrochemical stability and nonvolatility.