2025 AIChE Annual Meeting
(485f) Genome Engineering of Clostridium Acetobutylicum for Carbon-Neutral Isopropanol Production in a Synthetic Microbial Coculture
Authors
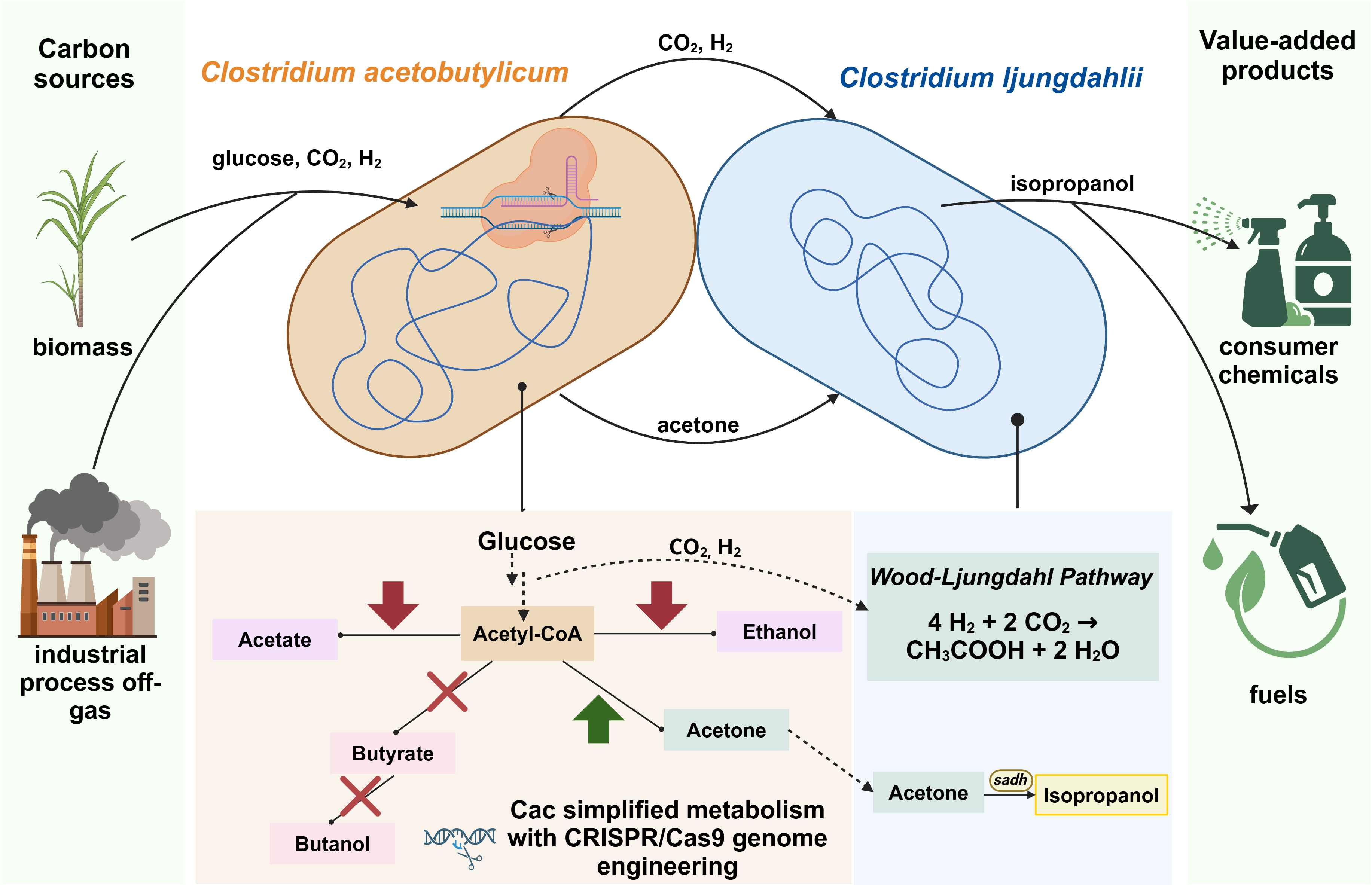
Genome engineering of Cac, including the deletion of competing pathways and enzymes to favor production of acetone, is our strategy. We employ several methods, including screening enzymes for acetone pathway production from multiple-copy plasmids to stable integration of constitutively expressed copies of the acetone operon in the Cac genome using CRISPR/Cas9. Through strong constitutive expression of key acetone pathway enzymes, particularly CoA transferase, we have seen marked increases in the acetone production in Cac monocultures. Our work demonstrates both synthetic biology and metabolic engineering strategies to maximize the reassimilation of CO2 to favor isopropanol production. While our work on this microbial coculture serves as an example of carbon-neutral production of isopropanol, these same strategies can be more broadly applied to carbon-neutral production of other desirable chemicals.
Supported by the U.S. Department of Energy ARPA-E project under contract AR0001505.