2025 AIChE Annual Meeting
(180l) Functionalized ZIF-67 Nanocomposite for Efficient Dye Adsorption from Wastewater
Abstract
Water pollution creates a significant threat to human health and the environment. Wastewater containing dyes is one of the major environmental concerns. There are huge effluents of dye-contaminated water from industries such as textiles, food manufacturing, pulp and paper, cosmetics, and pharmaceuticals annually. Azo dyes (those containing N=N bond) have widespread industrial use due to their chemical stability and vibrant coloration. Their complicated structure prevents these pollutants from being removed through conventional wastewater treatments, therefore making them endure challenging organic contaminants. Amaranth (Acid Red 27), which ranks among the most used industrial dyes, is extensively used in the textile industry. The widespread use of amaranth has resulted in multiple health problems, including allergic responses as well as respiratory difficulties and, potentially, tumor formation [1]. The rising demand for clean water due to the increasing population and industrial activities requires sustainable and efficient wastewater treatment systems that effectively eliminate harmful pollutants. Among the available Wastewater treatment methods, adsorption stands out as an effective tool for removing pollutants with economic viability [2].
This study utilizes Zeolite Imidazolate Frameworks (ZIFs), a sub-class of Metal-Organic Frameworks (MOFs), as adsorbents for the removal of amaranth from wastewater samples. Particularly, ZIF-67, type of ZIFs containing cobalt ions linked to imidazolate linkers in its zeolite-like cubic structure, that is used as the starting material. The structural design of ZIF-67 contains metal nodes with organic linkers and functional groups to function as a promising adsorbent for dye removal. Dye adsorption occurs through surface complexation mechanisms, ion exchange, and pore diffusion, proving that ZIF-67 is effective for wastewater treatment [3]. However, the aggregation and difficult dispersibility in aqueous media limit the application of ZIF-67 for adsorptive wastewater treatment. Functionalization/modification of ZIF-67 with other materials might overcome this limitation. Accordingly, ZIF-67 was functionalized with 4,4'-(9-fluorenylidene) dianiline (FEDA), a component with diamine attached to fluorenylidene core, forming a nanocomposite abbreviated as FEDA@ZIF-67. To the best of our knowledge, this is the first study reporting the synthesis of this nanocomposite. After the successful synthesis of the FEDA@ZIF-67 nanocomposite, its performance in removing the azo dye amaranth from wastewater samples was investigated.
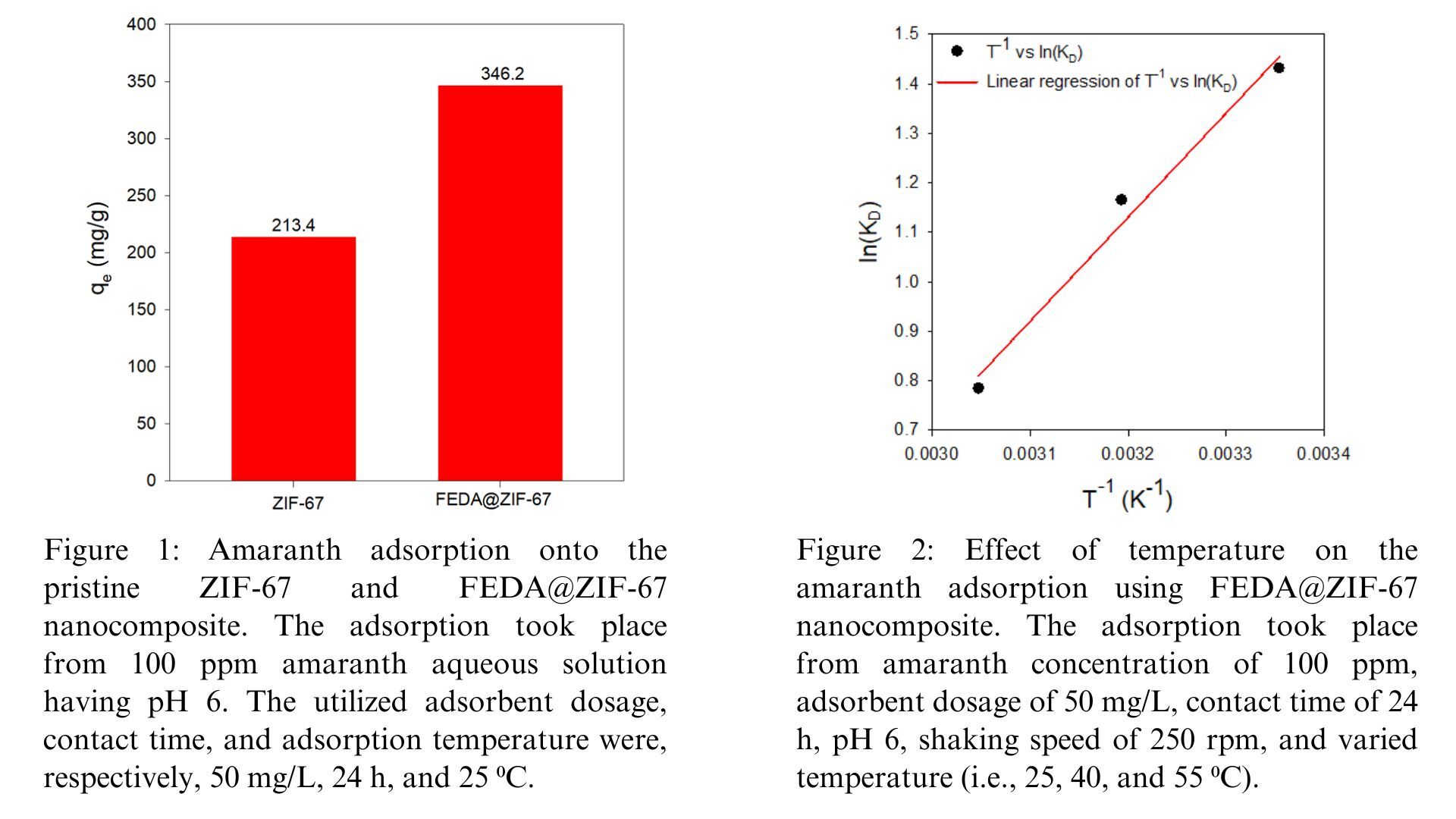
The synthesized FEDA@ZIF-67 nanocomposite outperformed the pristine ZIF-67, as shown in Figure 1. For instance, under the experimental conditions stated in the caption of Figure 1, the amaranth adsorption capacity onto the FEDA@ZIF-67 nanocomposite is about 60% higher than its adsorption capacity on the unmodified ZIF-67. More specifically, these adsorption capacities are 346.2 and 213.4 mg/g, respectively.
Besides the performance benchmarking results shown in Figure 1, the effects of process variables were also investigated. As an example, Figure 2 depicts the effect of temperature on the amaranth adsorption using the FEDA@ZIF-67 nanocomposite. The observed linear relationship between inverse temperature values (T-1) and natural logarithmic values of distribution coefficients (ln(KD)) estimates important thermodynamic parameters (i.e., enthalpy, entropy, and Gibbs energy changes). The negative Gibbs free energy (∆G) values at 298, 313, and 328 K indicate that the adsorption process occurs spontaneously. The equilibrium adsorption capacity (qe) decreases as temperature increases, indicating that the adsorption process occurs exothermically, which is further supported by the negative of the enthalpy change (∆H). Additionally, the negative entropy change (∆S) suggests a decreased randomness during adsorption at the adsorbent–solution interface. These results prove that FEDA@ZIF-67 adsorbs amaranth through a spontaneous and exothermic process, demonstrating enhanced efficiency when the system operates at lower temperatures.
Overall, the simple synthetic process and high adsorption capacity along with the high reusability performance of the FEDA@ZIF-67 nanocomposite make it a highly promising adsorbent for wastewater dye removal applications, emphasizing the significance of this study.
References
[1] K. Lin KunYi [Lin and W. C. Wu ChangHusan, “Efficient adsorptive removal of toxic Amaranth dye from water using a zeolitic imidazolate framework,” 2018.
[2] U. M. Ismail, M. S. Vohra, and S. A. Onaizi, “Adsorptive removal of heavy metals from aqueous solutions: Progress of adsorbents development and their effectiveness,” Jun. 15, 2024, Academic Press Inc. doi: 10.1016/j.envres.2024.118562.
[3] S. A. Ganiyu, M. A. Suleiman, W. A. Al-Amrani, A. K. Usman, and S. A. Onaizi, “Adsorptive removal of organic pollutants from contaminated waters using zeolitic imidazolate framework composites: A comprehensive and up-to-date review,” Sep Purif Technol, vol. 318, p. 123765, 2023.