2025 AIChE Annual Meeting
(66i) From Formate to Toluene: Unified Approaches in Catalyst Optimization for Hydrogen Storage and Release
Author
Comparative Strategies: Hydrides vs. Carriers
Building on these insights, we also investigate hydrogen production through both hydride-based and carrier-based approaches, focusing on electrochemical hydrogenation of toluene as a complementary pathway to formate systems. By collecting and analyzing literature-derived electrochemical kinetic (Tafel) parameters—encompassing activity, selectivity, catalyst composition, and loading—we can employ a simplified (0D) electrolyzer model to characterize performance. This bottom-up analysis obviates the immediate need for complex simulations, while subsequent surrogate modeling and targeted COMSOL-based sensitivity studies guide us in identifying and optimizing critical parameters, such as cost–activity trade-offs. This integrated framework aims to establish an optimized catalyst roadmap validated by existing benchmarks, providing actionable guidelines for catalyst design and optimization in hydrogen carrier technologies. Combining mechanistic elucidation, data-driven modeling, and a structured reverse-engineering process, our work contributes to advancing both fundamental understanding and practical implementation of efficient, durable hydrogen production systems.
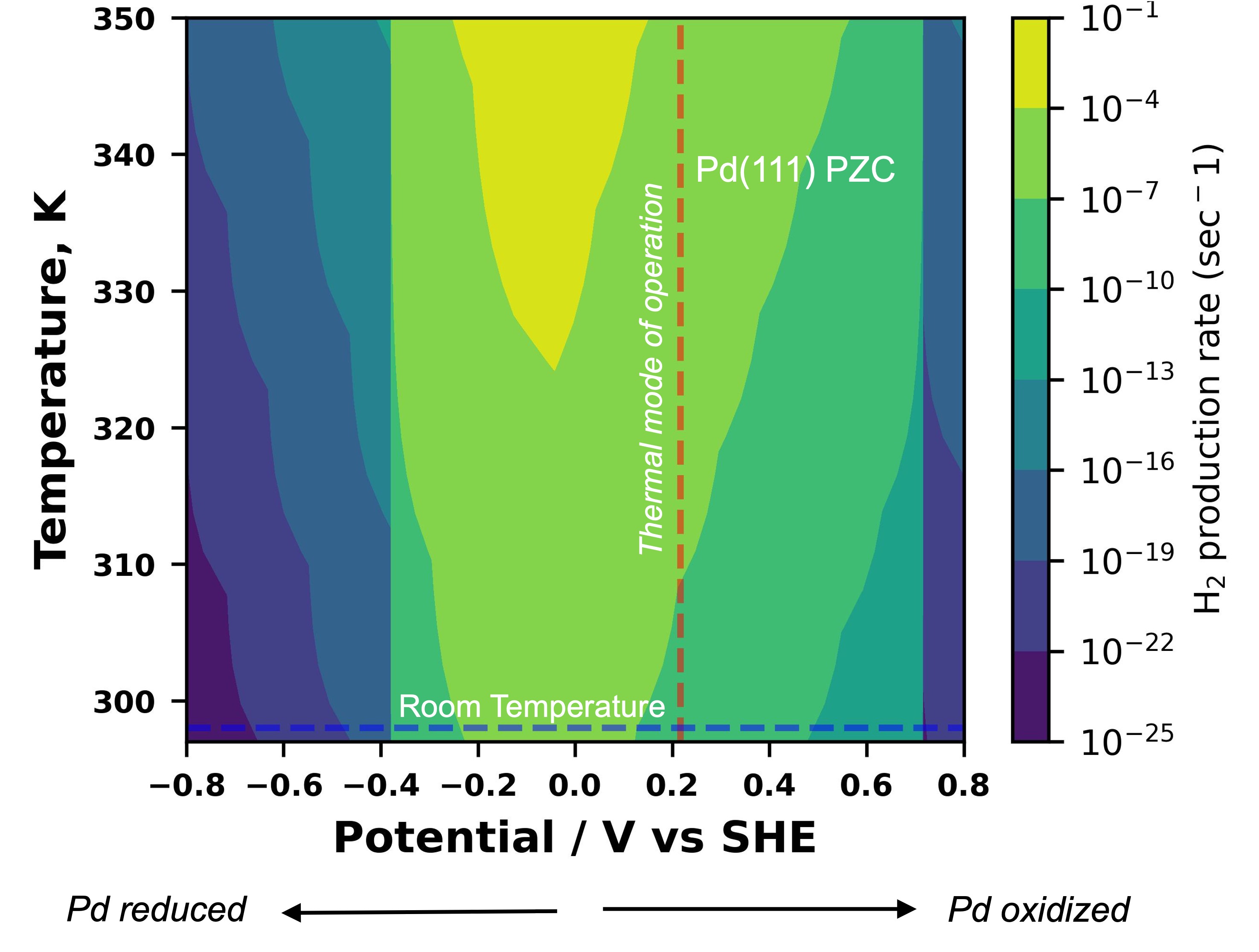
Figure 1. A heatmap for the variation of dehydrogenation rate (sec-1) against the operational choices of temperature and the electrode potential. the thermal mode of operation is denoted by the vertical red line (at the PZC of Pd), while the room temperature electrochemical route is shown via the horizontal blue line.