2025 AIChE Annual Meeting
(182ai) Fluorogenesis - Photoinduced Chromophore Dissociation Enables Aggregation-Driven Red Fluorescence
Authors
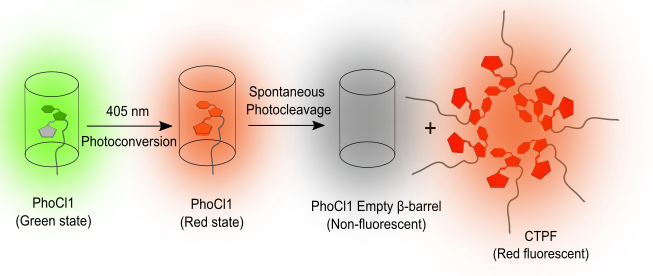
We engineered PhoCl1 with a 6xHis tag at its N-terminus to facilitate binding to Ni-NTA resin. Upon irradiation with 405 nm light, the CTPF dissociates and is released into the supernatant, exhibiting red fluorescence. The CTPF present in the supernatant was characterized through MALDI and SDS-PAGE which revealed the absence of impurities. We further performed intensive spectroscopic analyses, including absorbance and fluorescence spectra, lifetime measurements, and quantum yield and fluorescence anisotropy assessment to provide additional insights into the properties of the fluorescent fragment.
To elucidate the mechanism behind the observed red fluorescence, we hypothesized that the CTPF units aggregate which results in the exclusion of water from the chromophore environment, thereby enhancing fluorescence. Dynamic light scattering (DLS) and fluorescence anisotropy measurements supported this aggregation-based mechanism. Quantum mechanics/molecular mechanics (QM/MM) analyses further corroborated these findings. Additionally, we evaluated the fluorescence behavior of the CTPF under various environmental conditions, including changes in pH, NaCl concentration, and temperature.
These findings suggest a reconsideration of the previously proposed model that PhoCl1 loses its fluorescence upon light treatment and suggest an alternative model wherein the cleaved CTPF exhibits red fluorescence due to aggregation.