2025 AIChE Annual Meeting
(620g) Fluorescence Switching Sensor for Fentanyl and Fentanyl Analog Detection Using Graphene Quantum Dots and CB[7]
Authors
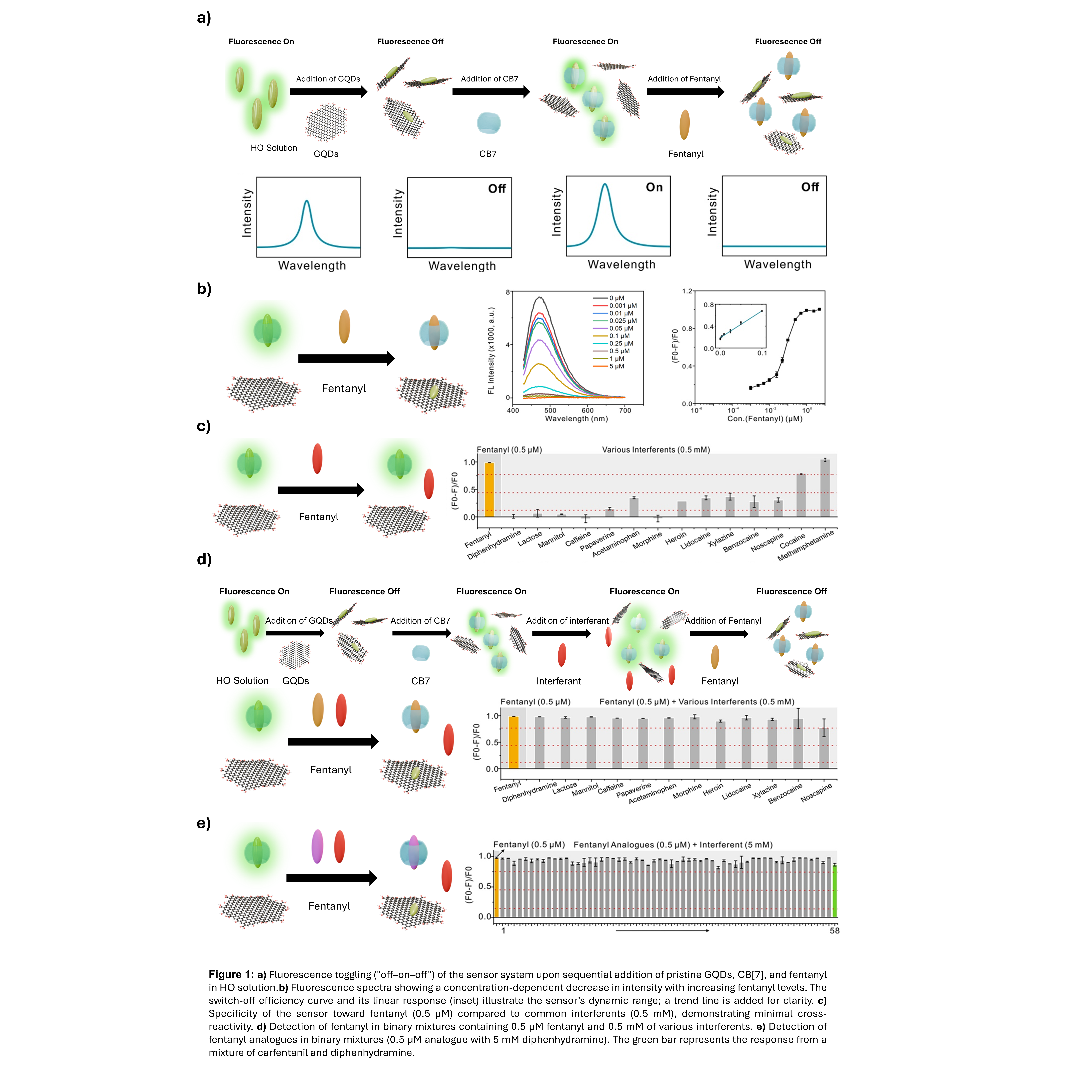
The sensing system operates through a dynamic host–guest interaction that modulates the fluorescence of HO. HO, a cationic fluorescent dye, strongly adsorbs to the negatively charged surface of GQDs, resulting in significant quenching of its emission through electrostatic and π–π stacking interactions. Upon introduction of CB[7], HO is competitively desorbed from the GQD surface due to the formation of a stable inclusion complex between HO and the CB[7] cavity. This desorption leads to a marked increase in fluorescence intensity. When fentanyl or structurally related analogs are introduced into the system, they bind to CB[7] with even greater affinity than HO and displace it from the cavity. The released HO re-adsorbs to the GQD surface and is quenched once again, generating a distinct "on–off" fluorescence response. This sequence—quenching by GQDs, rescue by CB[7], and displacement by the analyte—underpins the platform’s sensitive and reversible detection mechanism. The optimal sensor formulation consists of HO (0.25 μM), pristine GQDs (0.5 μM), and CB[7] (0.25 μM) in deionized water at pH ~5.8. GQDs were synthesized via a modified hydrothermal method and characterized by transmission electron microscopy (TEM) and zeta potential analysis, revealing an average particle diameter of 7.3 ± 1.2 nm and a surface charge of −21.0 ± 2.4 mV. CB[7] was prepared using standard synthetic protocols and confirmed to form stable complexes with HO via isothermal titration calorimetry and fluorescence titration, with a binding affinity on the order of 10⁶ M⁻¹.
Sensor performance was initially validated using model guest compounds with known CB[7] binding affinities, including 1-adamantanol, p-xylylenediamine, and hexamethylenediamine. Each of these guests effectively displaced HO from CB[7], resulting in measurable and reproducible fluorescence switch-off responses. The limit of detection (LOD) for these analytes ranged between 3.0 and 9.0 nM, confirming the high sensitivity of the system. Importantly, inclusion of GQDs was essential for achieving a low fluorescence background and maximizing signal contrast. In the absence of GQDs, displacement of HO from CB[7] produced only minor changes in fluorescence, underscoring the synergistic role of nanomaterial quenching in the overall mechanism. The sensor was then applied to fentanyl detection, where it displayed a dose-dependent decrease in fluorescence intensity across the 0.001–5 µM concentration range. The calculated LOD for fentanyl was 7.9 nM, a performance metric that compares favorably with existing field-deployable detection technologies. The system demonstrated excellent solution-phase stability, maintaining consistent fluorescence response profiles over a 20-day testing period. The sensor was further challenged with 19 commonly encountered interferents—including cutting agents, pharmaceutical compounds, and other illicit drugs—and maintained its responsiveness to fentanyl even in the presence of a 5 mM background concentration of interferent. Even at a 0.01 mol% fentanyl concentration in the presence of a 5 mM interferent background, a statistically significant fluorescence quenching response was observed
To assess generalizability, the platform was extended to a library of 58 fentanyl analogs. These included structural variants with substitutions at the acyl group, phenyl ring, and piperidine ring, as well as highly potent compounds such as carfentanil. Each analog was tested at 0.5 μM, and all produced strong fluorescence switch-off signals indicative of successful competitive displacement of HO from CB[7]. Even when analogs were tested in the presence of diphenhydramine (5 mM), the sensor reliably maintained its detection capability. This result confirms that the host–guest interaction between CB[7] and fentanyl-class molecules is sufficiently robust and generalizable to enable detection across a wide structural space. Unlike traditional immunoassay-based fentanyl test strips, which are highly target-specific and prone to false negatives with emergent analogs, this supramolecular sensor platform detects a broad spectrum of fentanyl and fentanyl analogs using a unified detection mechanism.
In conclusion, the HO/GQD/CB[7] supramolecular fluorescence switching sensor provides a sensitive, selective, and generalizable strategy for the detection of fentanyl and structurally diverse analogs in aqueous environments. The sensor achieves low nanomolar detection limits, within a few minutes response times, and excellent chemical stability, all without the need for covalent modification of the analyte or complex instrumentation. Its successful detection of 58 fentanyl analogs—including ultra-potent compounds like carfentanil—demonstrates a level of versatility and robustness not currently achievable with common method of fentanyl detection. By leveraging host–guest displacement and nanomaterial quenching, this system offers a practical, solution-based approach to addressing the challenges of synthetic opioid detection in forensic, clinical, and harm-reduction contexts. As the synthetic opioid landscape continues to evolve, such broadly responsive detection platforms will be essential in supporting public health and safety efforts on a global scale.