2025 AIChE Annual Meeting
(574d) Fluid Assisted Transformation of Fallopian tube Epithelial Cells
Author
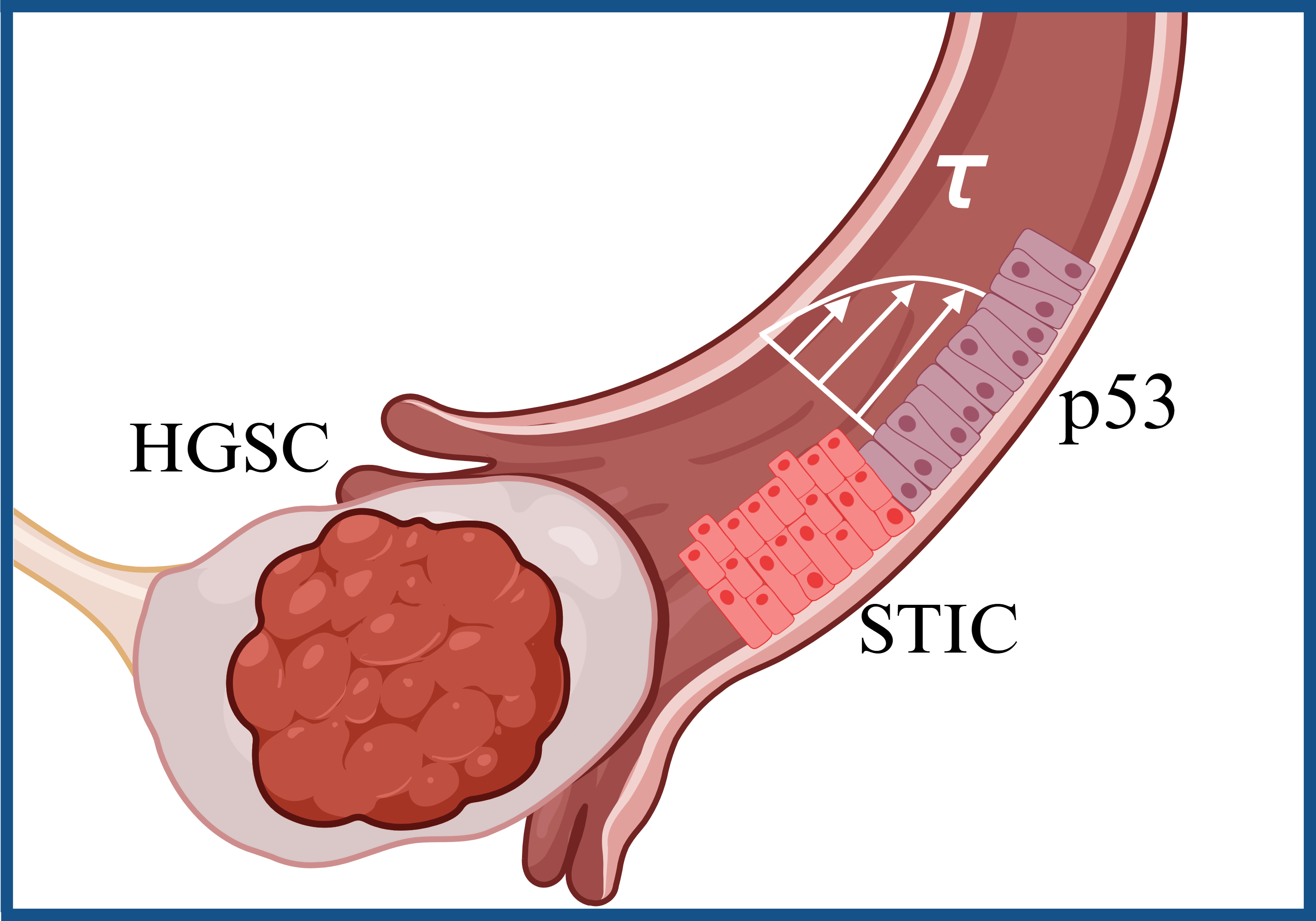
High grade serous ovarian carcinoma (HGSC) is the most common type and most lethal gynecologic cancer due to its late-stage diagnosis[1]. An established site of origin of the deadliest malignancy in women known as high grade serous cancer (HGSC) are the fimbriae[2,3]. The fimbriae are finger-like structures at opposite ends of the fallopian tube that connect the ovaries to the uterus. Unidirectional fluid flow in the fallopian tube plays an important role in gynecological health. The fimbriated ends experience varying levels of shear stress during ovulation, fertilization, and flow in the peritoneal cavity. Epidemiological studies have shown strong positive correlation between an increased risk of HGSC for women with higher lifetime ovulation[4]. Shear stress has been investigated in the context of in vitro fertilization that suggested the physiological range to be in the range of 0.007 to 0.7 dyne/cm2[5–7]. Currently, there are no studies investigating the effects of physiologically relevant mechanical stimuli such as shear stress on the origin of HGSC. Fallopian tube secretory epithelial cells (FTSECs) in the fimbriae undergo genetic and epigenetic transformations that lead to malignantly transformed cells that detach from the fimbriae spreading to peritoneal cavity around the abdomen and the ovaries. Therefore, the central hypothesis of this work is that fluid shear stress activates the transformation and dissemination of FTSECs. A custom built micro-physiological system (MPS) was utilized to stimulate FTSECs with shear stress . A viscous solution of methylcellulose and cell culture growth medium was perfused over the FTSEC over 24hrs to apply shear stress at 4 levels: 0.7, 1, 2, and 5 dyne/cm2. Shear stressed FTSECs revealed an increase of gene and protein expression of markers associated with pre-malignant serous tubal epithelial carcinoma (STIC) transformation. Specifically, STMN1 and CCNE1 increased up to four folds under shear stress stimulation of 5 dyne/cm2 for 24 hours in FT237 p53-/- cells. Further, RNA sequencing of FT237 at 5 dyne/cm2 demonstrated differentially upregulated genes associated with pathways such as MYC targets, TGF-beta signaling, and EMT, which are also activated in the metastasis of HGSC. Additional studies are underway to pinpoint the molecular mechanisms involved in shear stress mechanotransduction of FTSEC. This work will uncover the earliest changes that occur in the FTSEC as they progress towards HGSC, and therefore contribute to identification of early detection markers that can be utilized for improving HGSC cure rates. It will also provide an in-depth understanding of the pathogenesis of HGSC within the context of fluid dynamics of the fallopian tube.
References:
- Penn, C.A.; Alvarez, R.D. Current Issues in the Management of Patients With Newly Diagnosed Advanced-Stage High-Grade Serous Carcinoma of the Ovary. JCO Oncology Practice 2023, 19, 116–122, doi:10.1200/OP.22.00461.
- Zhang, S.; Dolgalev, I.; Zhang, T.; Ran, H.; Levine, D.A.; Neel, B.G. Both Fallopian Tube and Ovarian Surface Epithelium Are Cells-of-Origin for High-Grade Serous Ovarian Carcinoma. Nat Commun 2019, 10, 5367, doi:10.1038/s41467-019-13116-2.
- Shih, I.-M.; Wang, Y.; Wang, T.-L. The Origin of Ovarian Cancer Species and Precancerous Landscape. The American Journal of Pathology 2021, 191, 26–39, doi:10.1016/j.ajpath.2020.09.006.
- Fu, Z.; Brooks, M.M.; Irvin, S.; Jordan, S.; Aben, K.K.H.; Anton-Culver, H.; Bandera, E.V.; Beckmann, M.W.; Berchuck, A.; Brooks-Wilson, A.; et al. Lifetime Ovulatory Years and Risk of Epithelial Ovarian Cancer: A Multinational Pooled Analysis. JNCI: Journal of the National Cancer Institute 2023, 115, 539–551, doi:10.1093/jnci/djad011.
- Matsuura, K.; Hayashi, N.; Kuroda, Y.; Takiue, C.; Hirata, R.; Takenami, M.; Aoi, Y.; Yoshioka, N.; Habara, T.; Mukaida, T.; et al. Improved Development of Mouse and Human Embryos Using a Tilting Embryo Culture System. Reproductive BioMedicine Online 2010, 20, 358–364, doi:10.1016/j.rbmo.2009.12.002.
- Esteves, T.C.; Van Rossem, F.; Nordhoff, V.; Schlatt, S.; Boiani, M.; Le Gac, S. A Microfluidic System Supports Single Mouse Embryo Culture Leading to Full-Term Development. RSC Adv. 2013, 3, 26451, doi:10.1039/c3ra44453h.
- Ferraz, M.A.M.M.; Rho, H.S.; Hemerich, D.; Henning, H.H.W.; Van Tol, H.T.A.; Hölker, M.; Besenfelder, U.; Mokry, M.; Vos, P.L.A.M.; Stout, T.A.E.; et al. An Oviduct-on-a-Chip Provides an Enhanced in Vitro Environment for Zygote Genome Reprogramming. Nat Commun 2018, 9, 4934, doi:10.1038/s41467-018-07119-8.