2025 AIChE Annual Meeting
(253g) First Principles Studies of CO Electro-Oxidation at the Indium (hydroxy) Oxide/Platinum Three Phase Boundary
Authors
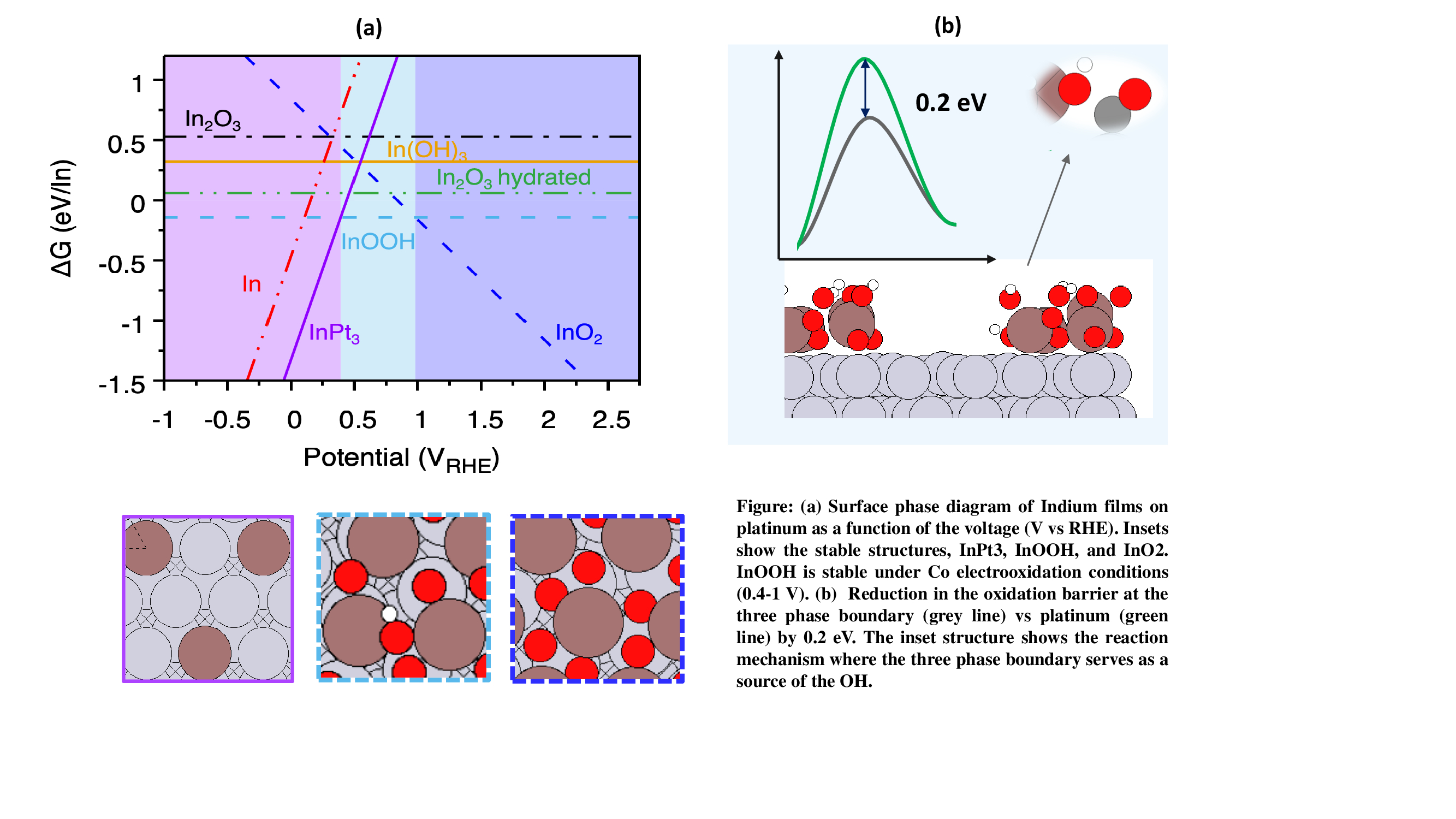
Although strategies such as reformate pretreatment and on-board separations exist to mitigate CO poisoning, considerable research has focused on developing CO-resistant electrocatalysts. While multimetallic alloys of platinum have demonstrated some ability to enable CO electro-oxidation, they suffer from low stability under electrochemical conditions. Recently, transition metal (hydroxy) oxides have emerged as promising catalysts for various electrochemical reactions. Experimental studies have synthesized ultra-thin hydroxy(oxide) metal films on platinum nanoparticles and evaluated their CO oxidation activities. Among many other metals, indium exhibited the greatest reduction in overpotential compared to pure platinum (0.15 V). In this work, we systematically investigate the origin of indium’s enhanced activity for CO oxidation using Density Functional Theory (DFT) calculations. Pourbaix analysis identifies an InOOH film on Pt as the most stable phase under reaction conditions. Kinetic analysis, in turn, reveals a distinctive bifunctional mechanism at the three-phase boundary, where the (hydroxy)oxide serves as a source of OH. Unlike pure Pt, where OH adsorption is hindered by CO poisoning, this catalyst facilitates oxidation, thereby lowering the effective activation barrier by 0.2 eV. These findings provide fundamental insights into the design of CO-tolerant electrocatalysts and highlight the potential of indium-based catalysts for enhancing fuel cell performance under practical conditions.