2025 AIChE Annual Meeting
(584am) A First-Principles Analysis of Carbon Materials As Catalysts for the Non-Oxidative Coupling of Methane Reaction
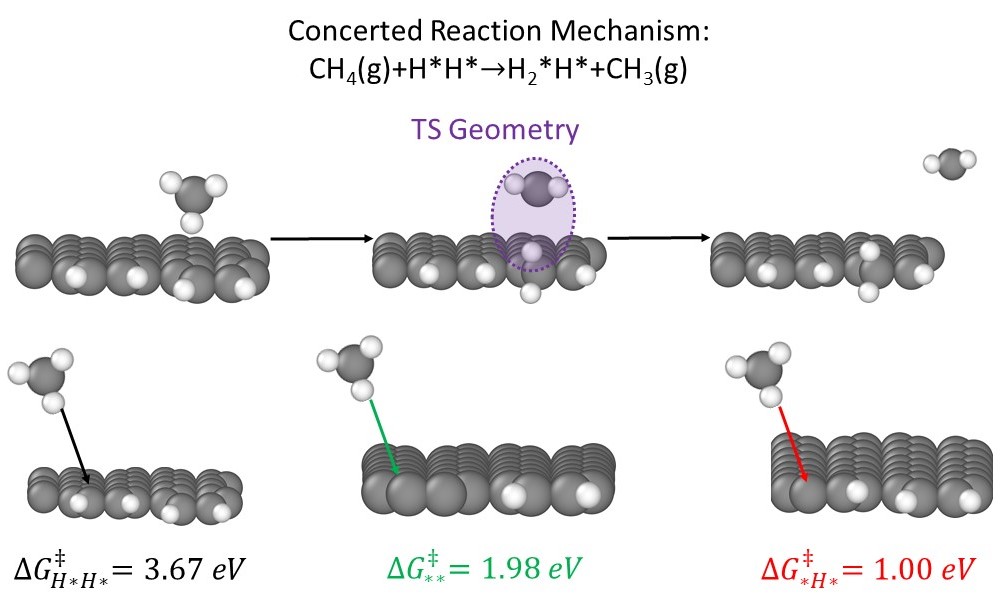
Ethylene (C2H4) is a feedstock for many industrial chemicals, from polyethylene to ethylene glycol. Current C2H4 production is dominated by naphtha steam cracking, an energy-intensive process which releases significant amounts of carbon dioxide (CO2). In this work, the Non-Oxidative Coupling of Methane (NOCM) reaction is studied as an alternative means of producing C2H4. NOCM has the potential benefits of being able to exploit methane (CH4) present in United States shale gas resources without a concomitant release of CO2. Previous work has demonstrated that carbon materials can serve as effective CH4 conversion catalysts, but the mechanism by which carbon promotes NOCM is unknown. To elucidate the molecular details of this reaction, we study methane activation on edge terminations of graphene – the “armchair” and “zigzag” configurations – as well as the graphene basal planes, as models of carbon active sites. We determine that byproduct hydrogen gas will adsorb to and passivate what would otherwise be dangling bonds on edge terminations of graphene via a surface phase diagram analysis. Despite this, the barrier to CH4 activation is still significantly lower on edge terminations compared to the basal plane. Effective NOCM barriers can be further reduced by a phenomenon involving hydrogen migration along the graphene edges to temporarily un-passivate these edges and produce transient dangling bonds which readily convert CH4. Microkinetic models suggest that the rate-limiting step for the NOCM process is hydrogen migration for edge models and methane activation for the basal plane. We close by briefly discussing the potential of Zeolite-Templated Carbon (ZTC) materials as alternative carbon allotropes to promote this chemistry.