2025 AIChE Annual Meeting
(524d) Field-Enhanced Ammonia Catalytic Cracking for Hydrogen Generation: A Multi-Scale Simulation Study
Authors
Qiang Li, University of Massachusetts Lowell
Yilang Liu, University of Massachusetts Lowell
Fanglin Che, University of Massachusetts Lowell
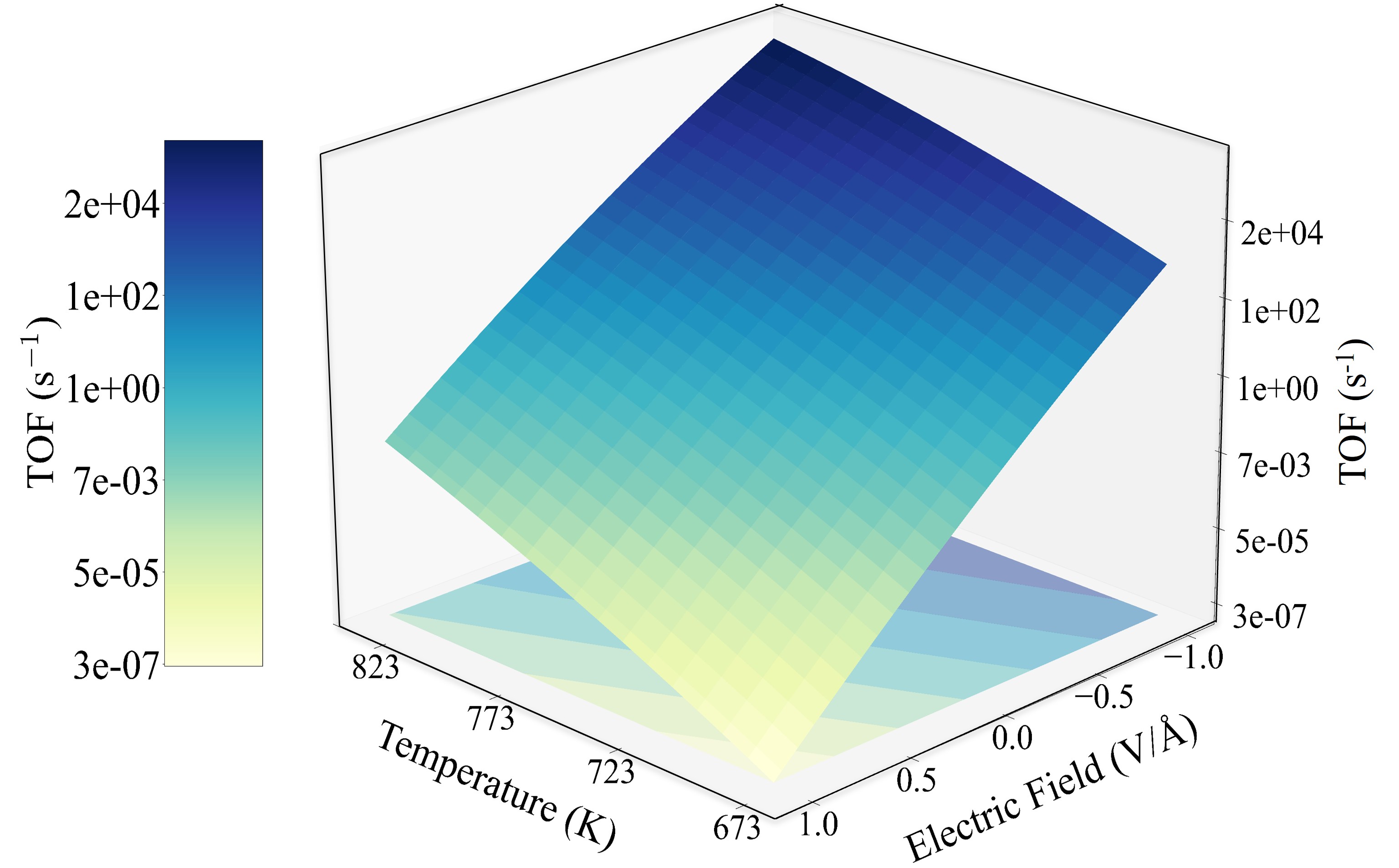
Ammonia decomposition is a promising method for on-site hydrogen generation, but conventional heating with ruthenium (Ru) catalysts requires high temperatures, limiting industrial viability. Applying strong external electric fields offers a solution, as NH3 decomposition is sensitive to field-dipole interactions. However, screening reaction conditions via trial-and-error is impractical due to high experimental costs. To address this, we developed a multi-scale simulation framework combining density functional theory (DFT) with microkinetic modeling (MKM). This approach provides mechanistic insights, identifies critical pathways, and optimizes conditions for field-enhanced NH3 decomposition over Ru catalysts. Our model (Figure 1) shows a significant increase in catalytic activity with a negative electric field. At 673 K, a field of -1 V/Å boosts the turnover frequency from 0.03 s⁻¹ to 1435.2 s⁻¹. This trend continues at higher temperatures; at 823 K, the negative field increases the turnover frequency four-fold compared to no field. Applying a -1 V/Å field also allows for a substantial temperature reduction, from 750 K to 586 K, while maintaining a turnover frequency of 5 s⁻¹. A sensitivity analysis reveals NH dehydrogenation over Ru(1013) as the rate-limiting step across various fields and temperatures. This multi-scale model offers crucial insights into field-enhanced catalysis, contributing to more sustainable hydrogen production methods. By leveraging electric fields, we can enhance catalytic performance without increasing catalyst complexity, making ammonia decomposition more viable for industrial applications.