2025 AIChE Annual Meeting
(453g) Exploring Structure-Property Relationships of 3D-Printable, Self-Healing Polyamide and Polyester-Amide Ionenes
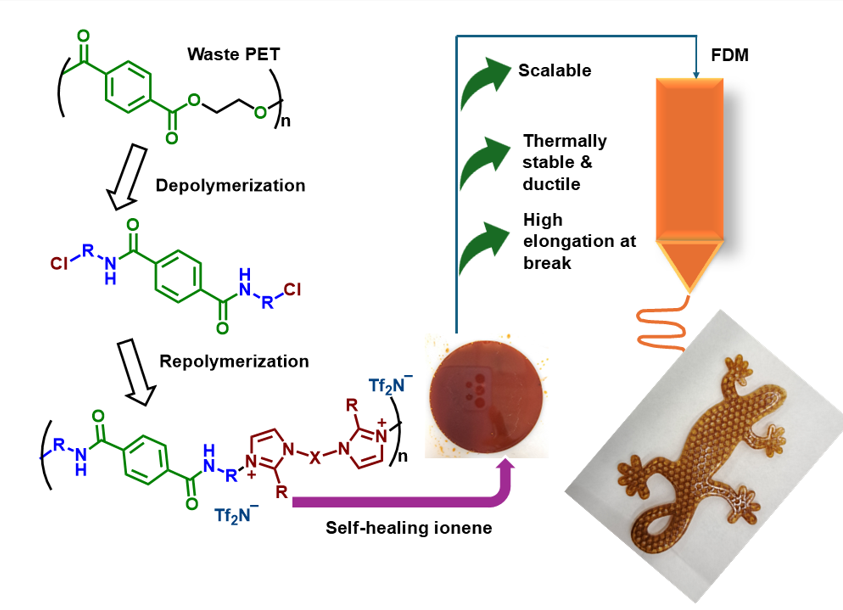
Ionenes represent a distinctive class of high-performance condensation polymers with broad applicability in electrochemistry, gas separation membranes, adsorbents, etc. Recently, these materials also emerged as a potential feedstock for 3D printing. Herein, we report the facile synthesis of polyamide (PA) and polyester-amide (PEA) ionene with a diverse set of alkanolamines and glycols having the terephthaloyl backbone. In parallel, bisimidazole derivatives with various linkers were synthesized and employed as nucleophilic building blocks. The resulting ionenes exhibited good thermal stability and intrinsic self-healing behavior above their glass transition temperatures, enabling their direct processability via fused deposition modeling (FDM) printing. Structural characterization revealed microcrystalline domains in the PA ionenes, while PEAs remained entirely amorphous. Rheological and mechanical analyses further demonstrated that the interplay between primary functional groups and bisimidazolium linkers plays a key role in governing the materials’ properties, either to be strong or stretchable.