2025 AIChE Annual Meeting
Exploring Efficiency of Cost-Effective Ferric Pt Bimetallic Catalysts
Experimentation and application of the Cooperative Redox Enhancement (CORE) mechanism (defined below) is currently limited to precious bimetallic systems, which are expensive and difficult to scale due to the scarcity of precious metals. By replacing a precious metal with a readily available element (Fe) while maintaining the catalytic rate, we can shorten development times, and clear pathways for large-scale applications in green technology. Herein, we vary electrodeposition time and substrate to show that a one minute deposition onto an electrochemically activated carbon cloth substrate produces the greatest current during CORE reduction-oxidation reaction testing.
Introduction:
Research teams at Lehigh and Cardiff University have recently documented the enhanced reaction rates of supported bimetallic catalysts, attributing it to a cooperative redox enhancement (CORE) mechanism (Daniel), where electrochemically connected metal nanoparticles act in tandem during a reduction-oxidation (redox) reaction. The potential of each nanoparticle, when connected, shifts to a middle value that accelerates the half of the reaction each metal type is superior at executing. However, precious metals such as gold and palladium used in CORE bimetallic catalysts are expensive and have serious negative environmental impacts (Mooiman). To this end, being able to use cost effective metals (Fe) in the CORE mechanism could accelerate research by decreasing costs and increasing availability of supplies, in addition to being a more accessible material for green technology in industry.
Results and discussion:
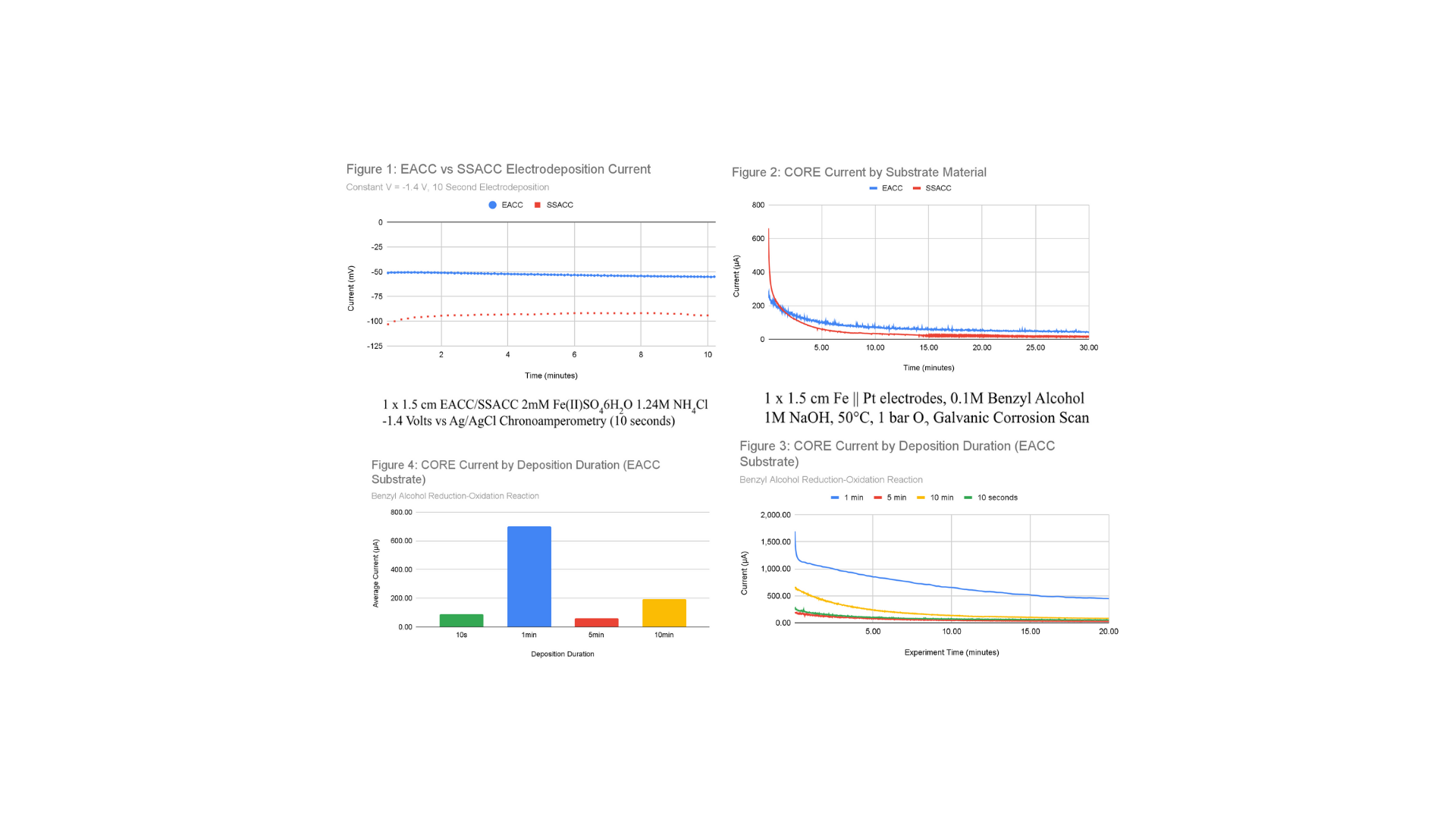
The two variables explored in this research were time of deposition and substrate material. The substrate material that produced the higher current was electrochemically activated carbon cloth (EACC), which was compared to single-side activated carbon cloth (SSACC). EACC was produced via redox reaction, applying 50 V for 15 minutes to a 1x1.5 cm carbon cloth sample in 2mM NH4Cl solution before reversing the voltage for 15 minutes. SSACC was procured in the form of Hydro-LAT 1400 Carbon Cloth with Hydrophilic Microporous Layer. Electrodeposition was performed to produce a 10 minute, 5 minute, 1 minute, 30 second, and 10 second sample on EACC substrate, and a 60 second, 30 second, and 10 second sample on SSACC substrate (Figure 1). The 10 second electrodeposition samples for SSACC and EACC were tested to determine the more effective substrate (Figure 2). During electrodeposition, SSACC showed a larger current than EACC during electrodeposition, but produced a lesser current by 227% during CORE testing. Further analysis of EACC substrates showed that the 1 minute electrodeposition EACC sample produced the greatest current during redox reaction testing by approximately 500% (Figures 3, 4). This data supports the morphology of the Fe deposited onto the EACC substrate for a 1 minute period being most effective at enabling the CORE mechanism to increase the efficiency of an Fe/Pt bimetallic catalyst.
Conclusions: Significantly more research is required to definitely determine the role iron can play in the CORE mechanism, but these preliminary results suggest that it is a viable alternative to the precious metals used in current CORE research. Further research will prove repeatability, test the effects of varying concentration of iron in the deposition solution, and use morphology scanning techniques such as SEM to determine the iron nanoparticle morphology on the substrate surface.