2025 AIChE Annual Meeting
(499b) Exploration of Size-Tunable Superparamagnetic Iron Oxide/Fluorescent Conjugated Polymer Composite Nanoparticles for Sentinel Lymph Node Biopsy
Authors
1. Introduction
Breast cancer metastasizes to other parts of the body through the lymphatic system. In most cases of metastasis, cancer cells remain in the first draining nodes in the lymphatic system, called as sentinel lymph node (SLN), making it an effective diagnostic target for metastasis. To find SLN for biopsy, integrating magnetic and fluorescent imaging capabilities is a promising approach, by which the position of SLN could be detected by magnetic probe preoperatively, followed by the minimum surgical resection guided by fluorescence.
Superparamagnetic iron oxide (SPIO) particles could be used as a label for identifying SLN by magnetic field. Besides, conjugated polymer nanoparticles [1] have been investigated as a new class of fluorescent materials because of their high biocompatibility and photostability. However, although composite nanoparticles using SPIO and conjugated polymer have been reported for different purposes, their large size [2] or non-spherical morphology [3] was not suitable for SLN detection. It has been reported that the size of nanoparticles significantly affects their accumulation in the SLN [4]. Moreover, the reported value of the optimal size varies by paper, presumably due to different experimental conditions and differences in the tumor/lymphatic microenvironment in experimental animals. Therefore, considering the heterogeneity of tumor and lymphatic systems between patients, as well as the diverse application of SLN biopsy for different types of cancer, precise and tailor-made tuning of particle size is required for the application of magnetic and fluorescent nanoparticles (MFNs) for SLN biopsy.
2. Purpose
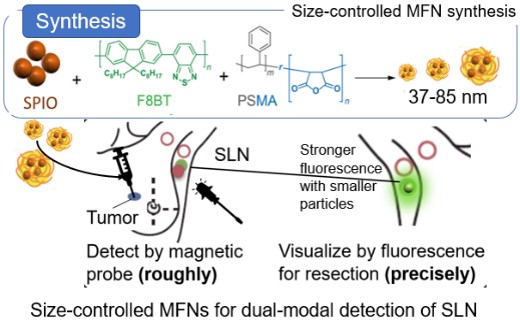
In this work, we aim to develop size-tunable and biocompatible MFNs using SPIO and fluorescent conjugated polymer poly(9,9-dioctylfluorene-alt-menzothidiazole) (F8BT) to achieve precise and low-invasive SLN biopsy. (Fig. 1) The size of synthesized MFNs was controlled by the concentration and mass ratio of SPIO / F8BT. By integrating magnetism and fluorescence into the size-tunable nanoparticle platform, the development of a less invasive SLN detection method for the diagnostics of breast cancer metastasis is expected.
3. Experimental
- Synthesis and characterization of MFNs
F8BT and poly(styrene-co-maleic anhydride) (PSMA) were dissolved in tetrahydrofuran (THF) at varied concentrations (0.5, 0.25, and 0.125 mg/mL) with a mass ratio of F8BT to PSMA as 4:1. SPIO nanoparticles (5 nm in diameter) were dispersed in THF, and then mixed with the F8BT/PSMA solution at a mass ratio of SPIO to F8BT as 2:1 and 1:1. 1.0 ml of mixed solution was dropped into 20 mL pure water under ultrasonication and mechanical stirring. After THF removal by vacuum drying, and subsequent purification by ultracentrifugation, the size and morphology of the obtained MFNs were characterized by transmission electron microscopy (TEM) and dynamic light scattering (DLS). The fluorescent and magnetic properties of MFNs were evaluated using a fluorospectrometer and a vibrating sample magnetometer (VSM).
- Surface modification of MFNs
Amine-terminated polyethylene glycol (PEG, 5000 Da) conjugation to MFNs was performed by utilizing carbodiimide cross-linking reaction between carboxyl groups on the MFNs' surface and amine groups at the end of the PEG chains. The MFNs solution in 20 mM HEPES buffer (pH 8) with 0.05 % Tween20 was reacted with 20 molecules/nm2 of amine-terminated PEG, 20 molecules/nm2 of 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC/HCl), and 20 molecules/nm2 N-hydroxysuccinimide (NHS) for the surface area of MFNs. After the reaction solution was stirred overnight at room temperature, the particles were washed with pure water three times by ultracentrifugation. The PEG modification was confirmed by Fourier transfer infrared spectroscopy (FT-IR).
- Evaluation of the effect of MFNs size on lymph node accumulation in the mouse model
For in vivo SLN imaging by fluorescence, 40 μL of PEG-MFNs dispersion (0.5 mg Fe/mL) in phosphate buffered saline was injected into a mouse (Balb/c, f, 7-10 weeks) footpad subcutaneously, following to the incision of skin in the median line under isoflurane anesthesia. 1 hour after the PEG-MFNs injection, fluorescent images of mice were obtained using an in vivo imaging system (IVIS), with an excitation filter at 460 nm and an emission filter at 540 nm. In addition, T2-weighted 3D MRI imaging was performed to visualize the distribution of PEG-MFNs. Fluorescent signals from the extracted lymph nodes (popliteal lymph node: PO) were evaluated by IVIS after sacrificing the mice.
4. Results and discussion
- Synthesis and characterization of MFNs with different sizes
MFNs were synthesized by varying the concentration and the mass ratio of SPIO to F8BT. The MFNs displayed uniform spherical structures containing multiple SPIOs per particle. From the DLS measurements and TEM observation, it was confirmed that MFNs (37-85 nm) with spherical morphology were successfully synthesized. The size of the MFNs increased as the polymer concentration increased and/or as the SPIO/F8BT ratio increased. The higher polymer concentrations increase the particle growth rate during nanoprecipitation, resulting in a larger polymer matrix [5]. On the other hand, it was suggested that higher SPIO content allows more SPIO to be encapsulated within each nanoparticle, resulting in larger particle sizes.
The surface of MFNs was further modified with PEG to render biocompatibility. The successful PEG modification was confirmed by FT-IR spectra of MFNs after the modification, which showed a peak at 1170 cm-1, indicating C-O stretching of the ether of the PEG chains.
- Evaluation of the fluorescent and magnetic properties of synthesized MFNs
The fluorescent and magnetic property of MFNs was evaluated by the fluorospectrometer and VSM. Compared with pure F8BT and SPIO nanoparticles, the fluorescence and magnetic properties of MFNs decrease by about 50%, respectively. The decrease in fluorescence could be due to quenching from interactions between F8BT and SPIO, such as energy transfer. It was suggested that the reduced magnetization was caused by changes in surface magnetic anisotropy, resulting in disordered surface spins and weaker magnetic response. The mass magnetization of the synthesized MFNs was quantitatively measured by VSM to be 2 to 5 emu/g, which is consistent with previously reported values of fluorescent polymer/SPIO composite nanoparticles [6]. For fluorescence, significant differences were observed between MFNs with different SPIO: F8BT ratios, whereas no significant differences were found among samples with the same ratio. For magnetic performance, despite slight variations, no significant differences were observed, indicating that particle size had minimal impact on magnetic properties. These results demonstrated that we successfully achieved size-controlled synthesis of MFNs while maintaining consistent magnetic properties and fluorescence characteristics.
- Fluorescence/magnetic dual-modal imaging of mouse lymph nodes using size-controlled MFNs
The potential of MFNs as a probe for SLN detection for biopsy was demonstrated by subcutaneous injection into the mouse footpad, followed by IVIS and MRI to evaluate Small (37 nm), Middle (60 nm), and Large MFNs (85 nm) accumulation in the PO. 1 hour post-injection, fluorescent signals from PO on the injected side were detected by IVIS regardless of the size of the MFNs. T2-weighted 3D MRI also revealed signal reduction in the same node, confirming SPIO accumulation. These results demonstrated the applicability of synthesized MFNs for fluorescent/magnetic resonance dual-modal imaging. Fluorescence intensity of extracted PO from the injected side was further evaluated by analyzing IVIS images to investigate the effect of MFNs' size on their accumulation. It was revealed that Small MFNs had 1.6-fold higher fluorescence intensity than those given by Middle or Large MFNs, demonstrating enhanced accumulation of smaller nanoparticles. These results were consistent with the previous report that the smaller nanoparticles diffused more rapidly from the injection site to the lymphatic vessels, resulting in higher accumulation in the nearest lymph node [7]. These results suggested that our proposed method can work as a platform to control the size-dependent transport of MFNs to SLN.
5. Conclusion
Size-tunable MFNs were developed using SPIO and F8BT via a nanoprecipitation method. The resulting MFNs exhibited fluorescence and magnetic properties. Upon accumulation in SLN, they showed strong fluorescence and enabled clear visualization. Moreover, size variation influenced the lymph node imaging performance: smaller MFNs tended to accumulate more efficiently and produce stronger fluorescence signals in SLN, suggesting improved imaging sensitivity. Combined with their magnetic characteristics, MFNs also generated signal reduction in T2-weighted MRI, demonstrating their potential as dual-modal probes for SLN detection. These features suggested that the developed MFNs could enable less invasive and more accurate SLN detection for the diagnosis of breast cancer metastasis.
6. Acknowledgement
This study was supported by Advanced Research Infrastructure for Materials and Nanotechnology in Japan of the Ministry of Education, Culture, Sports, Science and Technology.
7. References
[1] C. Pan et al., Angew Chem. Int. Ed, 52, (2013), 10775-10779 [2] P. Howes et al., J. Am. Chem. Soc, 132, (2010), 9833-9842 [3] N. Arias-Ramos et al., Pharmaceutics, 13, (2021), 12581. [4] Podutwar, A. A. et al., JPRI, (2021), 64–74.[5] Y. Lin, et al., RSC Adv., 7, (2017), 55957–55965. [6] Z. Shen et al., ACS Nano, 11, (2017), 10992–11004. [7] Oussoren, C. et al., Biochim Biosphys Acta, 1328, (1997), 261–272.