2025 AIChE Annual Meeting
(52d) An Experimental Model of Formic Acid Adsorption on Copper Electrocatalysts
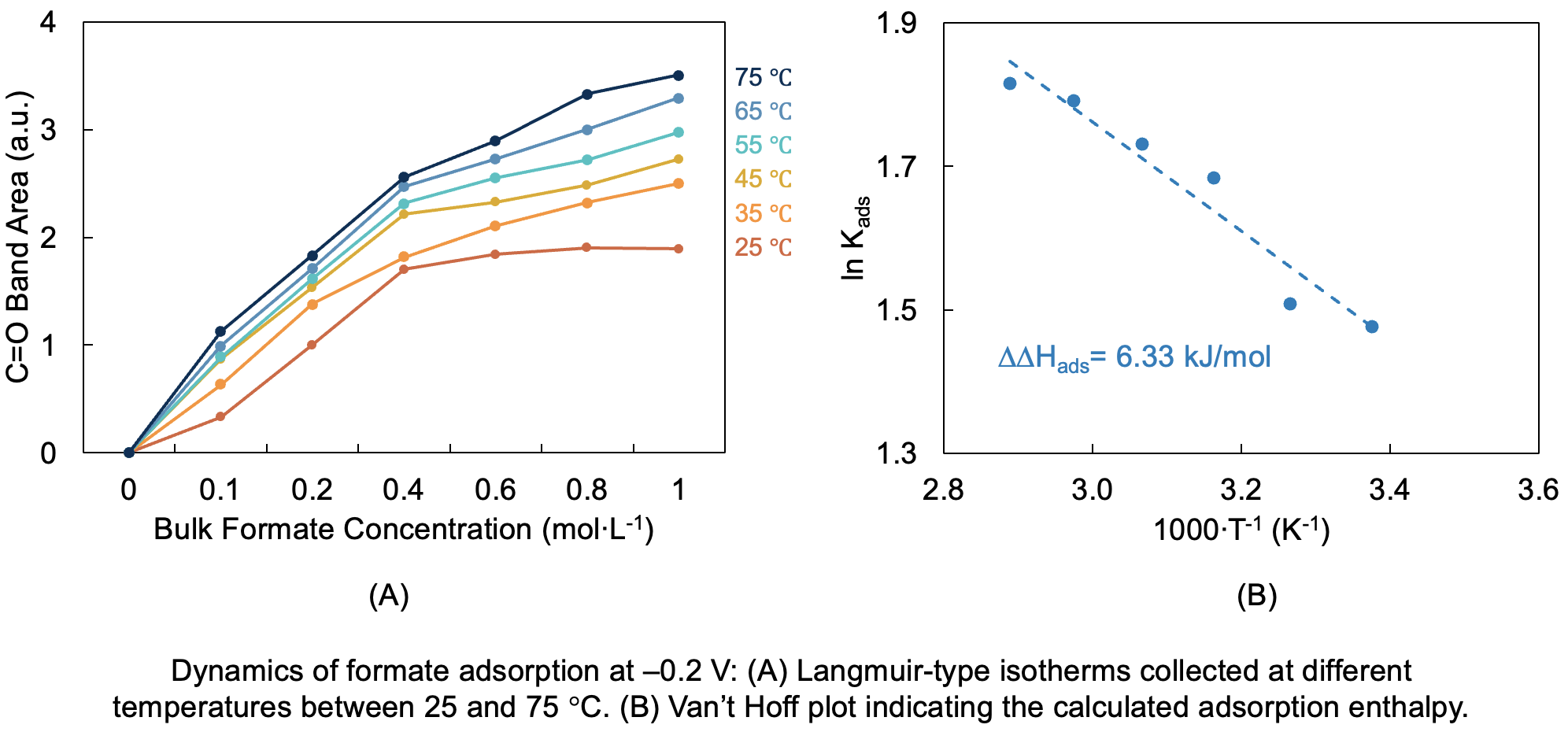
Polarization of copper electrodes in the presence of formic acid led to the formation of a band centered at 1710 cm-1, which was attributed to the C=O stretching mode of adsorbed formate. We showed that, at –0.2 V, the coverage of this anion follows a Langmuir-type isotherm for all temperatures between 25 and 75 °C (Fig. A). Higher temperature led to increased formate coverage for a given bulk concentration, which allowed us to estimate a positive DDH = 6.33 kJ/mol associated with the surface replacement of adsorbed electrolyte species by formate (Fig B). Further kinetic studies and isotherms collected at different potentials suggest that there is slightly more formate adsorbed at more positive potentials for a given temperature. Our results suggest that quantitatively understanding the binding strength of formic acid to charged electrodes is key to better design electrocatalysts under operating conditions that favor formic acid oxidation.