2025 AIChE Annual Meeting
(179i) Evaluation of Dispersion and Aggregation Behavior of Amine-Modified Silver Nanoparticles Based on Affinity between Organic Solvent and Modifier
Authors
The nanoparticle was silver with a diameter of 8 nm, and the solvent was toluene or methanol. The modifier was dodecylamine (C12A) or amphiphilic amines consisting of an alkyl chain (C) and an ethylene glycol chain (EG). The affinity was varied by changing the length of the modifier chain. The volume fraction of the modifier in the modifier layer was 0.5. The affinity between the solvent and the modifier was evaluated using the Flory-Huggins interaction parameter. When the interaction parameter is less than 0.5, the affinitiy is high and the nanoparticles will disperse, whereas when it is greater than 0.5, the affinity is low and the nanoparticles will form aggregates. The interaction parameter was calculated from the Hansen solubility parameter (HSP). The HSP is represented in terms of dispersion, polarity, and hydrogen bonding. The HSP of the solvent was estimated from the literature, and that of the modifier was estimated from the group contribution method.
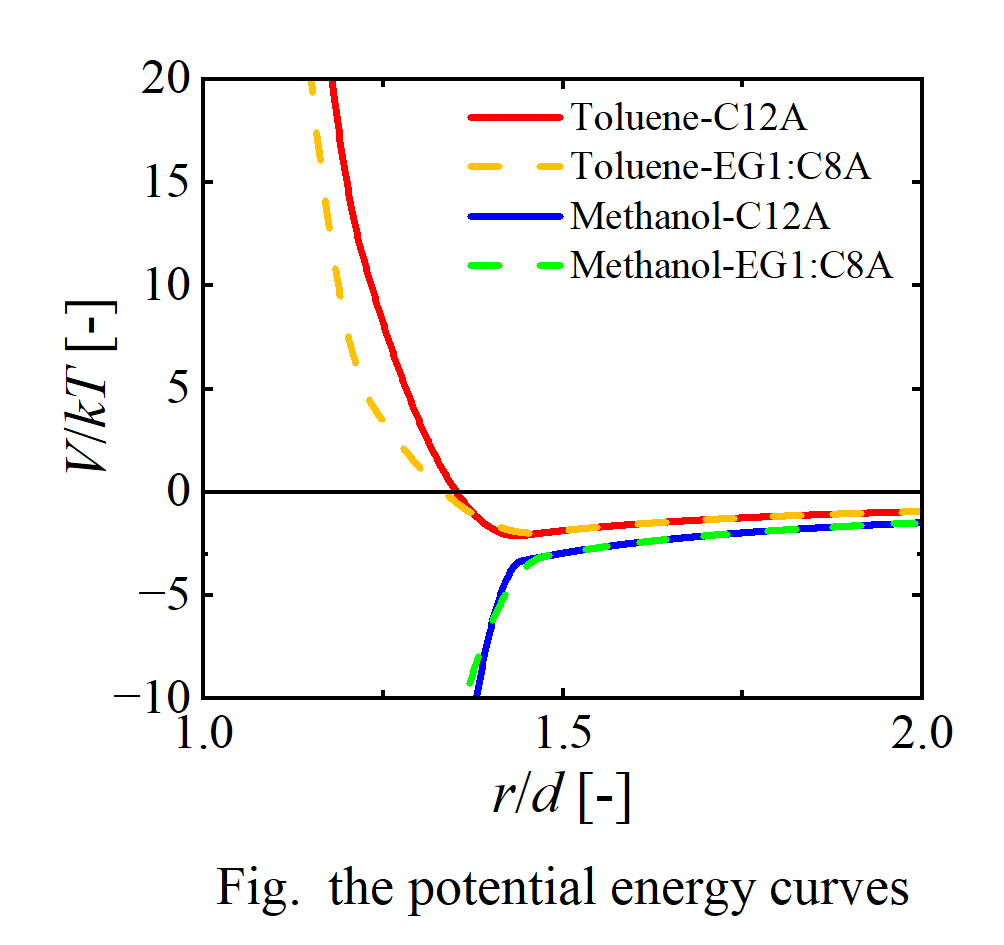
The higher the ratio of ethylene glycol chains, the higher the interaction parameters of polarity and hydrogen bonding of the modifier. This result indicates that the estimation of HSP based on the structure of the modifier is an appropriate representation of the structure. The affinity between C12A and toluene was high based on the interaction parameter. The affinity of modifier with methanol was high when the ratio of ethylene glycol chain in the modifier was high. The dispersion and aggregation behavior of nanoparticles predicted by the interaction parameter was consistent with previous experimental results. Therefore, it was suggested that predicting dispersibility by the HSP estimation can be applied. Furthermore, the potential energy curve between two nanoparticles was used to predict the dispersion state of nanoparticles. The figure shows the potential energy curves between two nanoparticles for different solvents and modifiers. k is Boltzmann’s constant, r is the distance between particle centers, T is the temperature, and V is the interaction energy between the nanoparticles. The interaction is a sum of the van der Waals interaction and the interactions induced by the mixing free energy and the elastic repulsion between modifiers. The affinity between the solvent and the modifier contributes to the interaction term of the mixing free energy. In the case of toluene, a potential well, i.e., a mimimum in the potential energy curve, existed and its depth was shallow. Therefore, it is predicted that the particles can be dispersed in toluene and aggregate in methanol. This result is consistent with the prediction based on the interaction parameter and the previous experimental results.
This method can be useful in selecting appropriate modifiers for dispersing particles in various solvents.