2025 AIChE Annual Meeting
(222f) Evaluating Shear-Induced Protein Denaturation in Injection Devices Using Flow-Resolved CFD-DEM Coupling
Authors
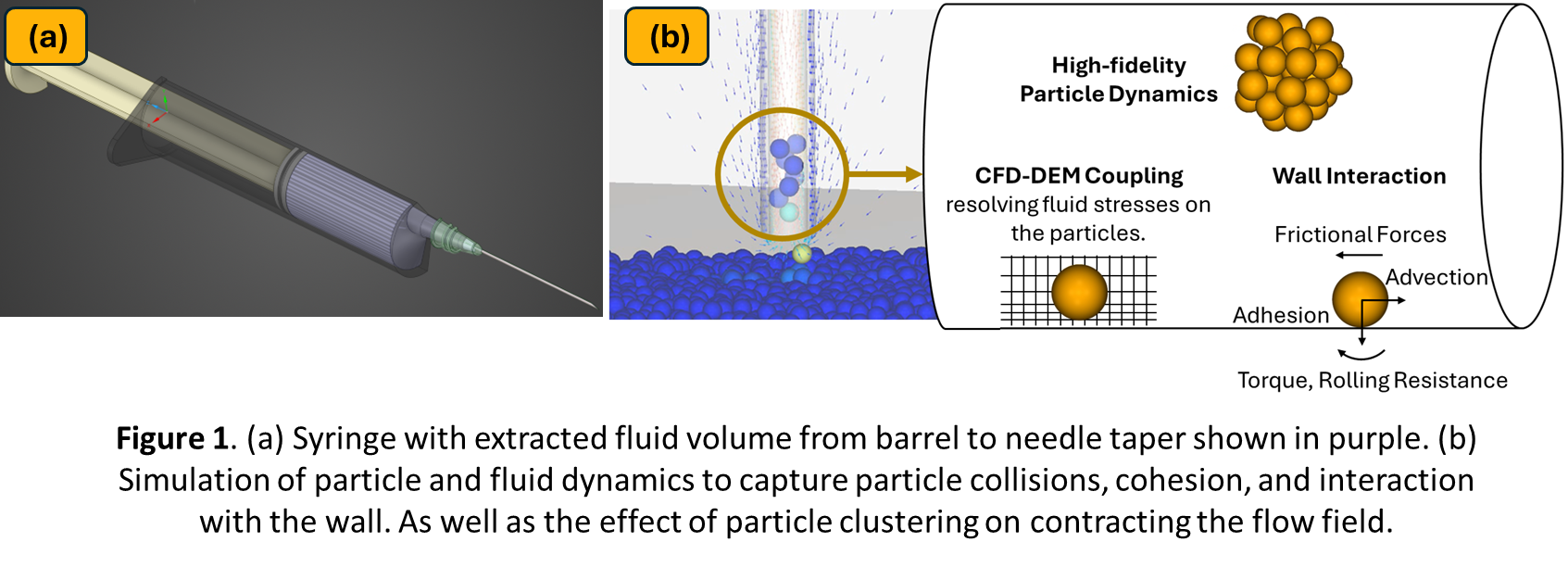
Coupled computational fluid dynamics and discrete element method (CFD-DEM) simulations provide a numerical approach to capture fluid-particle interactions at the microscale, and GPU acceleration further speeds the modeling of such small particles, enabling resolution of complex multiphase dynamics. The simulation domain focuses on the syringe lumen, from the barrel to the needle tip (Figure 1-a). The carrier fluid flow is solved using Ansys Fluent, with moving‑boundary conditions imposed by plunger kinematics. At each time step, the fluid velocity and pressure gradients are communicated to a GPU‑accelerated DEM solver (Ansys Rocky), where collisions and interactions of discrete particles are modeled to capture shear‑induced aggregation and breakup. This two‑way coupling resolves the flow around every particle, enabling quantification of flow filed changes because of particle clustering and interaction with individual surfaces.
Accurate representation of protein‑like particles requires calibration of material interaction properties. We employ a vertical suction‑pipe test under controlled flow conditions to tune particle rolling resistance and particle-wall friction (Figure 1-b) [2]. Additionally, the model setup is parameterized to evaluate different what‑if scenarios and understating of the particulate behavior. Together, these capabilities enable in-silico testing of the protein breakup during injection.
A parametric study was conducted by varying plunger velocity, fluid viscosity, and inter‑particle cohesion strength. Simulations show that narrow constrictions adjacent to the syringe wall serve as shear‑rate hotspots, with peak shear rates rising at higher injection speeds. Also higher inter‑particle cohesion delays breakup and denaturation but favors the formation of large clusters that risk device clogging, whereas lower cohesion leads to more uniform dispersion at the expense of excessive fragmentation. By fully resolving the fluid around individual particles and capturing cohesion dynamics, our CFD-DEM framework delivers insights into shear environments within drug delivery systems. As a predictive tool, it can guide formulation strategies (selecting excipients and viscosity modifiers), optimize needle design (taper, lumen diameter to minimize shear hot spots), and inform injection protocols (controlling plunger kinematics). This work showcases advantages of GPU‑accelerated implementations, providing a virtual platform to experiment and design safer, more effective drug administration devices.
References:
[1] Dharmaraj, Vishnu L., et al. "Rheology of clustering protein solutions." Biomicrofluidics 10.4 (2016).
[2] Wasserfall, Jacob G., Corné J. Coetzee, and Chris J. Meyer. "A submerged draw down test calibration method for fully-coupled CFD-DEM modelling." Frontiers in Chemical Engineering 6 (2024): 1376974.