2025 AIChE Annual Meeting
(512c) Ethanol Vapour-Induced Morphological Influences on the Water Barrier Performance of PLA Films: A Multi-Component Study
Authors
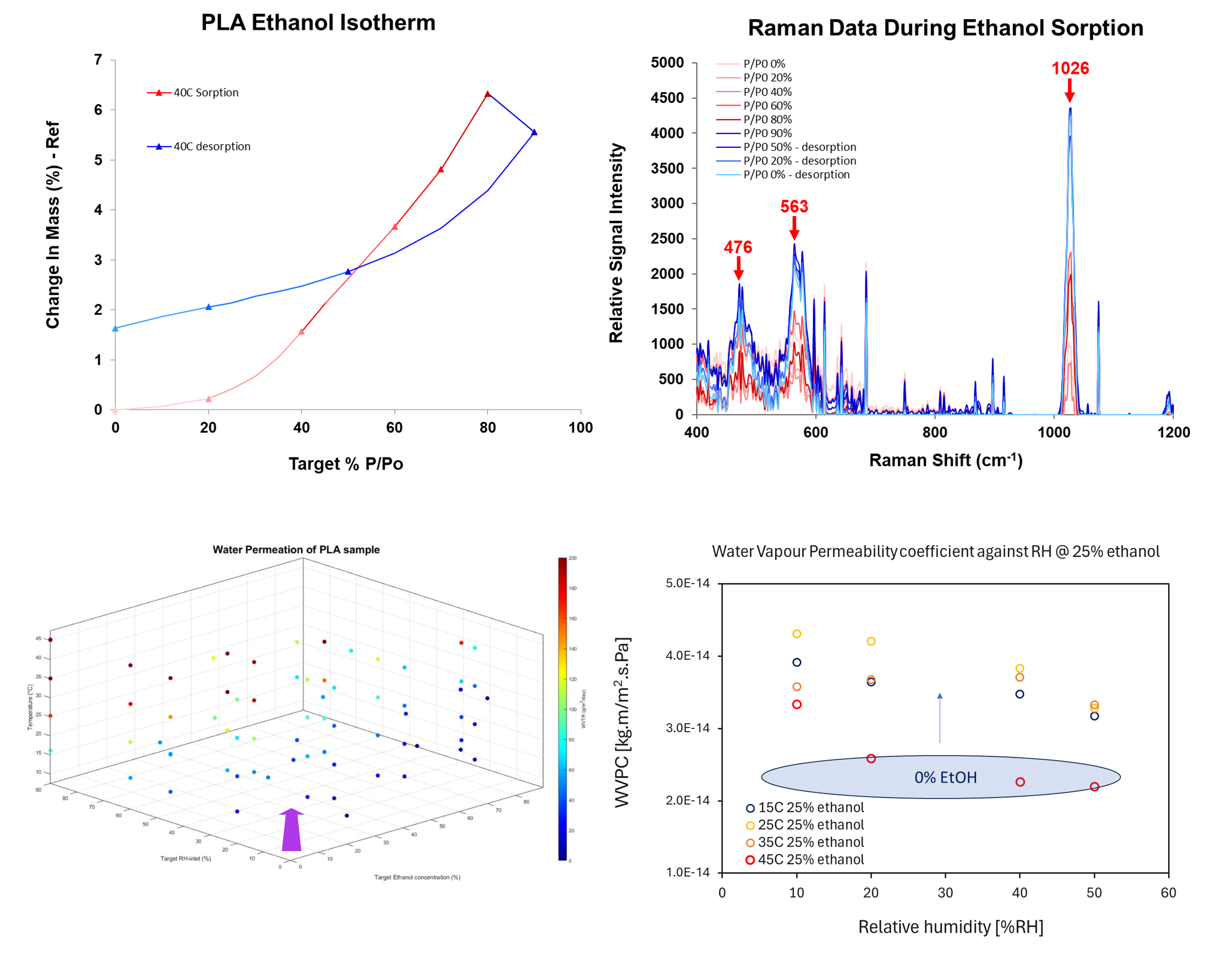
Preliminary results are consistent with those reported by Auras et al.2 where the water vapour permeability coefficient (WVPC) decreases through PLA with temperature, which was attributed to the relaxation of the rigid amorphous fraction (RAF) present at the boundaries of crystalline and amorphous domains. When the WVPC is analysed in the presence of 25% ethanol, we observe an overall increase in the WVPC (~40-60%) and a shift in the temperature at which the RAF contributes to a decrease in permeability (from 35 °C to 45 °C). This shift in the temperature effects is also seen in Iniguez-Franco et al.3 where the addition of an ethanol fraction of 25% resulted in a reduction of the glass transition temperature from >50 °C to >43 °C. The reduction of the Tg was attributed to solvent-induced crystallinity over long periods, whereas in this study it is likely to be a swelling effect over the smaller time scales. Investigations into the partition coefficients revealed a decrease (~25%) in the water partition coefficient with increasing ethanol concentrations, suggesting that ethanol decreases the solubility of water in PLA which results in a lower permeability. Studies by dynamic vapor sorption revealed a binary-effect whereby the presence of both humidity and ethanol vapor increased the solubility of the latter, and shifted the sorption isotherms to increase uptake at lower partial pressures. A combined DVS and in-situ raman study revealed the vapor induced swelling and crystallization (VIC) that occurs in PLA when exposed to ethanol vapor and humidity, with raman shifts at 476, 563, and 1026 cm-1 gradually increasing in intensity with vapor sorption suggesting swelling of the polymer and the maintaining intensity after vapor desorption suggesting a prolonged or irreversible morphological change based on VIC.
This study presents significant permeation and dynamic vapor sorption data on PLA water vapor permeation, stability and morphology changes under real-world conditions.
References:
- Sonchaeng, U. et al. Poly(lactic acid) mass transfer properties. Prog. Polym. Sci. 86, 85–121 (2018).
- Auras, R. A., Harte, B., Selke, S. & Hernandez, R. Mechanical, Physical, and Barrier Properties of Poly(Lactide) Films. J. Plast. Film Sheeting 19, 123–135 (2003).
- Iñiguez-Franco, F. et al. Concurrent solvent induced crystallization and hydrolytic degradation of PLA by water-ethanol solutions. Polymer 99, 315–323 (2016).