2025 AIChE Annual Meeting
(668c) Equilibrium and Non-Equilibrium Insights on the Direct Air Capture Performance of K-MER and 13X in Humid Environments
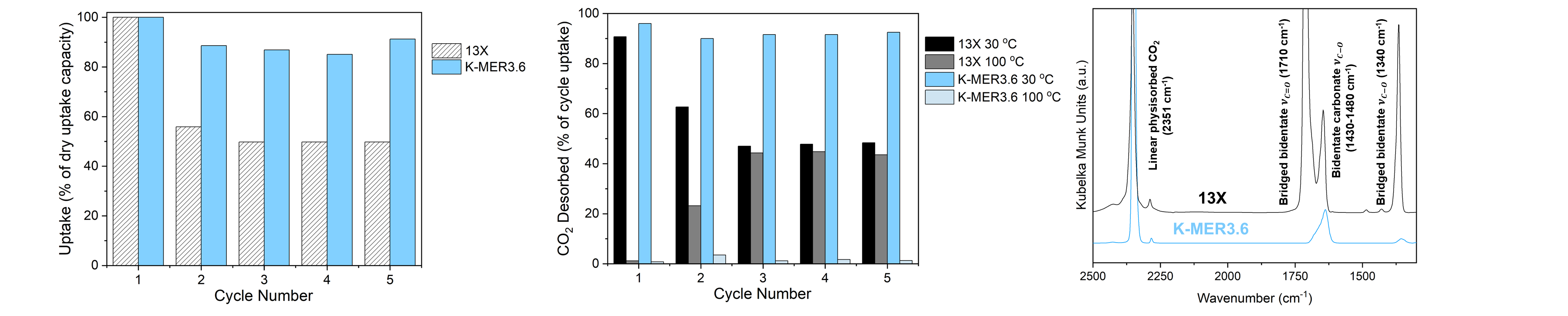
However, a recent breakthrough4 has demonstrated that certain zeolites, including K-MER (potassium-exchanged merlinoite), contain double 8-member ring (D8MR) subunits that have privileged sites for CO2 capture even in the presence of water vapor at high CO2 concentrations under equilibrium conditions. This finding highlights a new opportunity for zeolite design for humid CO2 capture and opens questions pertaining to (1) the concentration-dependence of this phenomenon, and (2) the performance of zeolites such as K-MER under the dynamic (i.e., non-equilibrium) operation representative of a DAC process. The work we present in this talk will address these two questions. Herein, we characterize the performance of K-MER under humid DAC concentrations and benchmark its performance to 13X (Na-FAU), a widely studied zeolite for CO2 capture. Through dynamic column breakthrough (DCB) adsorption and breakthrough studies, we validate the results of this previous work under 100% CO2, 5% RH conditions for K-MER at higher Si/Al ratio. However, we demonstrate that the equilibrium behavior observed at 100% CO2 does not hold at 400 ppm at 5% RH, 30 ºC (CO2:H2O: ~0.2 mol:mol).
Despite this finding, we will demonstrate that K-MER is still able to retain >90% of its dry CO2 uptake capacity throughout 5 cycles, in stark contrast to 13X (~50%), even under only moderate temperature swing regeneration of 100 ºC. Further, we will show how K-MER regenerates 90% of the adsorbed CO2 species using only a 30 °C purge with dry air over multiple cycles, in contrast to the 50% regeneration achieved under the same conditions for 13X. Desorption measurements from thermogravimetric analysis paired with insights from diffuse reflectance infrared spectroscopy (DRIFTS) studies show that the improved humid capture performance in K-MER can be attributed to weaker CO2 site strengths that are agnostic to water presence as well as greater water regeneration at moderate temperatures (100 °C). Finally, we also introduce the concept of an “nth cycle” test where humid CO2 is allowed to flow until water breakthrough, after which the cycling regeneration protocol is applied, and humid CO2 is again flowed until only CO2 breakthrough. This experiment seeks to estimate the minimum capacity drop that may be expected from a sorbent due to full water accumulation in the bed without requiring the ~100s if not ~1000s of cycles that would be needed to achieve water saturation of the entire bed. Collectively, these results highlight the importance of characterizing both equilibrium and non-equilibrium phenomena in binary adsorption in zeolites and set the stage for future studies on MER zeolites to elucidate the CO2 and H2O concentration-dependent performance of K-MER in capture applications.
(1) Intergovernmental Panel on Climate Change, I. Climate Change 2023 Synthesis Report, 2023.
(2) Direct Air Capture - Energy System. IEA. https://www.iea.org/energy-system/carbon-capture-utilisation-and-storag….
(3) Fu, D.; Davis, M. E. Toward the Feasible Direct Air Capture of Carbon Dioxide with Molecular Sieves by Water Management. Cell Rep. Phys. Sci. 2023, 4 (5), 101389. https://doi.org/10.1016/j.xcrp.2023.101389.
(4) Lee, H.; Xie, D.; Zones, S. I.; Katz, A. CO2 Desorbs Water from K-MER Zeolite under Equilibrium Control. J. Am. Chem. Soc. 2024, 146 (1), 68–72. https://doi.org/10.1021/jacs.3c10834.