2025 AIChE Annual Meeting
(114d) Enhancing Ethylene Production Via Side Chain Engineering of Anion-Exchange Ionomers for CO2 Electrolysis
Authors
Young In Song - Presenter, Korea Institute of Science and Technology
Jung Kyu Kim, SungKyunKwan University
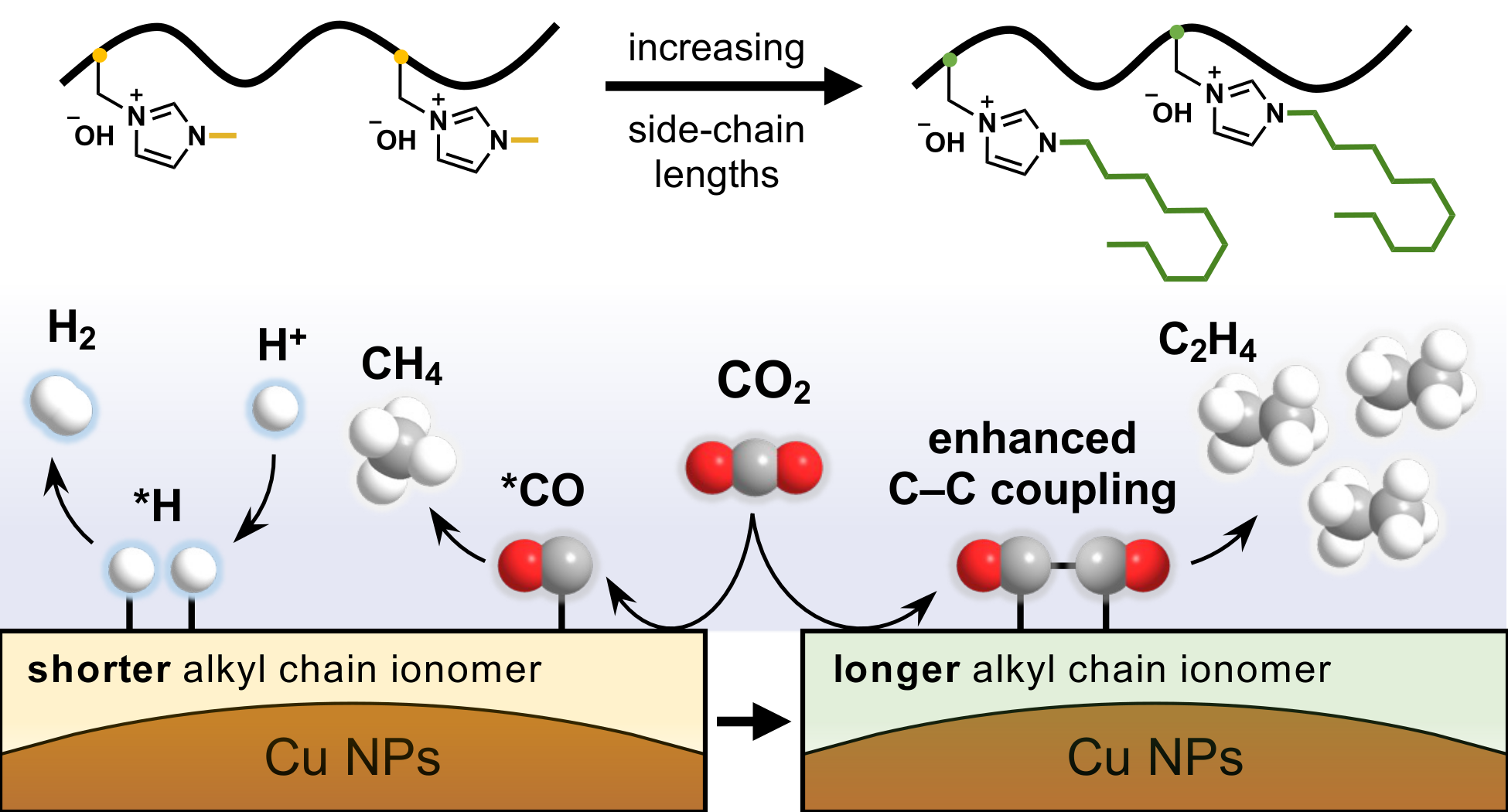
This study investigates how alkyl side chain length in imidazolium ionomers (CnH2n+1 where n = 1, 4, 10, 16) governs the activity and selectivity in Cu-catalyzed CO2 reduction reaction (CO2RR). Varying alkyl chain length enables precise control over reaction pathways. C2H4 selectivity peaks with the n-decyl ionomer (n = 10), which shows a 59.9% increase compared to the methyl analog. Density functional theory calculations reveal that longer side chains stabilize *2CO intermediates, promoting a C–C coupling reaction. In situ Raman and attenuated total reflectance–surface-enhanced infrared absorption spectroscopy (ATR–SEIRAS) demonstrate competing effects between decreasing local electric field and modification of interfacial water structure with increasing side chain length. At the same time, longer side chains reduce ionic conductivity due to declining ion-exchange capacity, increasing interfacial resistance and limiting current density. The highest C2H4 selectivity with the n-decyl ionomer reflects a balance between intermediate stabilization and transport limitations. The optimized n-decyl ionomer further achieves –209.5 mA cm−2 partial current density and 52.4% Faradaic efficiency for C2H4 production at 3.95 V with a moderately active Cu catalyst, highlighting its potential for industrial applications. This work advances the fundamental understanding of ionomer structure-performance relationships in electrochemical systems and provides generalizable design principles for CO2 conversion and related catalytic processes.