2025 AIChE Annual Meeting
Enhancing the Energy Efficiency of Lithium-ION Batteries through the Optimization of Nickel-Cobalt-Manganese Electrodes
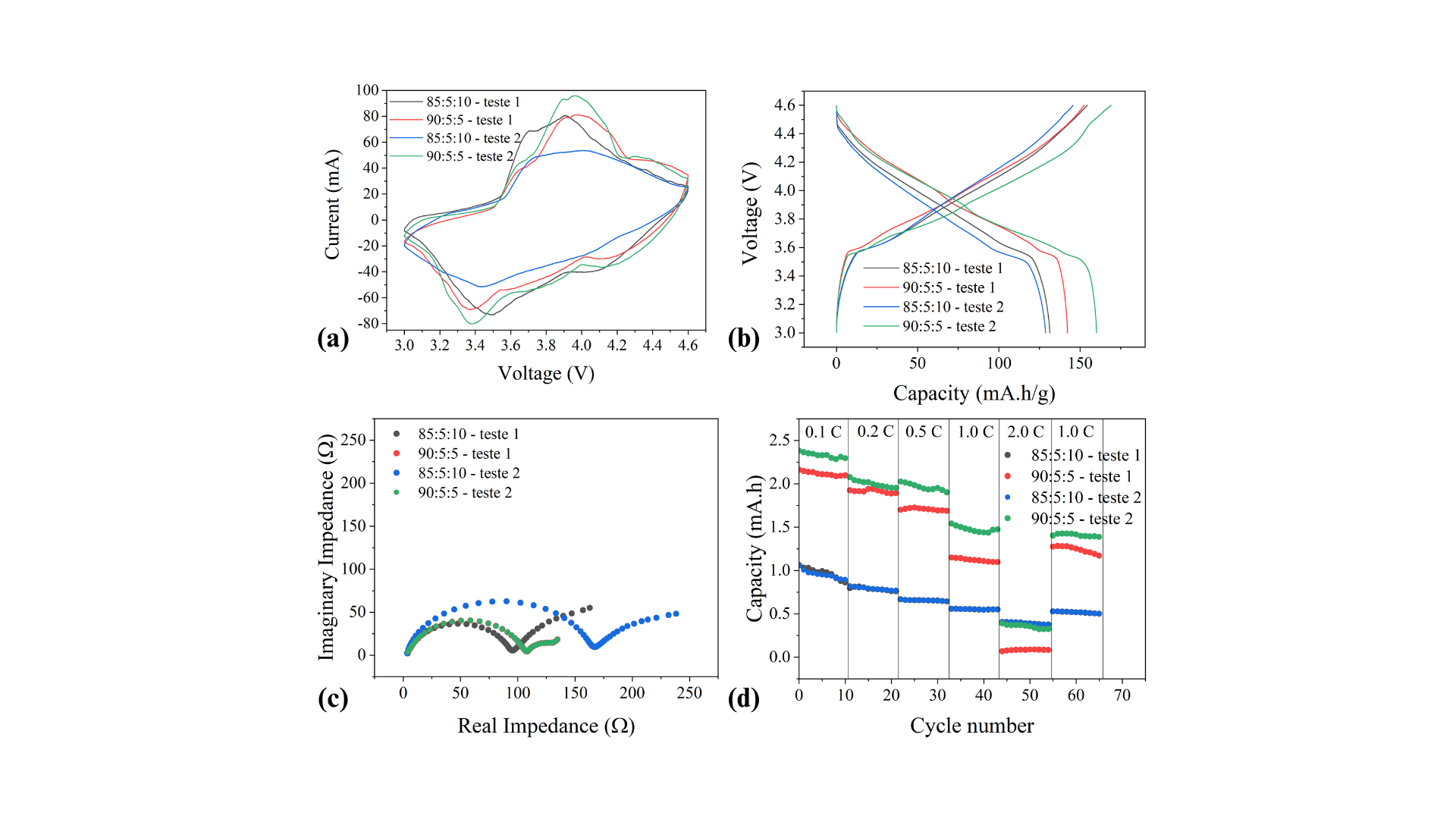
Four slurry formulations were prepared with active material:binder:conductive additive ratios of 85:5:10, 90:5:5, 90:2.5:7.5, and 92.5:2.5:7.5, using N-methyl-2-pyrrolidone (NMP, GELON) as the solvent. The coatings were applied at a thickness of 200 µm, dried at 60 °C, and their electrochemical performance was evaluated using coin cells assembled from the prepared electrodes. After electrode preparation, calendering was performed to the maximum compaction limit. The formulation with the highest active material content achieved greater compaction degrees and, consequently, higher densities. In terms of electrochemical performance, the best results were obtained for the 85:5:10 and 90:5:5 formulations, with the latter showing the highest specific capacity (~150–160 mA·h/g) and low charge-transfer resistance. Meanwhile, the 85:5:10 formulation exhibited lower performance and higher impedance. All cells showed a capacity drop at high charge rates but demonstrated good recovery when returned to 1.0 C. In addition, these cells were able to withstand over one thousand complete charge–discharge cycles.
The results demonstrate that electrode formulation plays a critical role in the performance of NCM-based lithium-ion batteries. Increasing the proportion of active material improved electrode compaction and density; however, the best electrochemical performance was achieved by the 90:5:5 formulation, which allowed for a higher active material content without increasing interfacial resistance, meaning the slurry maintained strong adhesion to the substrate. By optimizing the active material–binder–conductive additive ratio and enhancing electrode compaction, it is possible to enhance both the mechanical and electrochemical properties of electrodes, contributing to the development of more efficient lithium-ion batteries. These improvements enable greater charge storage within a smaller volume and extend battery cycle life, an essential step toward enabling reliable renewable energy storage and advancing the transition to a sustainable energy system.