2025 AIChE Annual Meeting
(193a) Enhanced Thermochemical CO?/H?O Splitting Via B-Site Fe-Mn Synergy in Pr0.4Sr0.6Mn1-YFeyO? (y=0.2, 0.4, 0.6, 0.8, 1) Perovskite
Authors
Solar energy is widely regarded as a sustainable alternative to fossil fuels, helping to reduce environmental harm. Among its various applications, concentrated solar power (CSP) offers distinct advantages over solar photovoltaic (PV) systems. Unlike PV, which only captures a portion of the solar spectrum and operates intermittently during daylight, CSP harnesses the full solar spectrum and can provide continuous energy availability. To ensure uninterrupted power generation, CSP systems incorporate thermal energy storage (TES), which stores excess solar heat during peak sunlight hours for later use during nighttime or adverse weather conditions. Several TES methods have been explored, including sensible, latent, and thermochemical storage systems. A particularly promising approach involves the thermochemical splitting of H₂O/CO₂ via a two-step cyclic process using non-stoichiometric metal oxides, producing hydrogen (H₂) or carbon monoxide (CO) as renewable fuels.
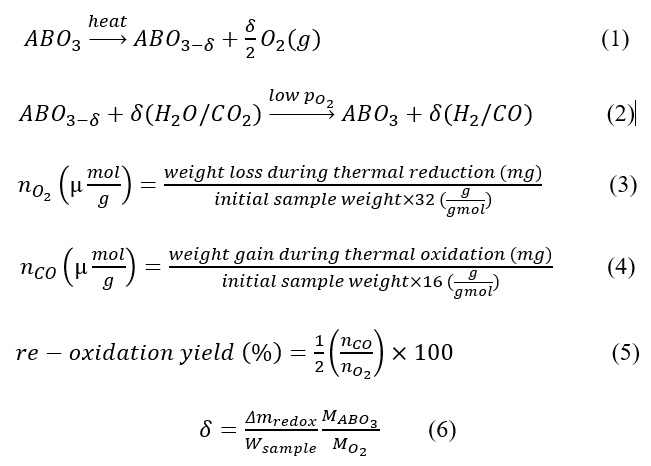
Perovskites are a class of materials with the general formula ABO₃, where A is a large alkali or alkaline earth metal cation (12-fold coordinated) and B is a smaller transition metal cation (6-fold coordinated), forming an octahedral oxygen framework. The ideal perovskite structure adopts a cubic geometry (space group Pm3m), though distortions often lead to orthorhombic or rhombohedral configurations. The Goldschmidt tolerance factor (t) helps predict the structural stability of perovskites by correlating ionic radii with possible space groups. Doped perovskites (AA'BB'O₃) incorporate substituents at the A and B sites to enhance functionality. In a two-step thermochemical cycle (shown in equation 1 and 2), these materials undergo solar-driven thermal reduction at high temperatures, forming oxygen-deficient perovskites (ABO₃₋δ, where δ represents oxygen non-stoichiometry). The reduced material then reacts with H₂O or CO₂, producing H₂ or CO while regenerating the original perovskite structure (ABO₃), thus completing the cycle.
The 1st reaction (i.e. thermal reduction) is endothermic and favored at higher temperatures whereas, the 2nd reaction (i.e., re-oxidation) is exothermic and favored at low oxygen partial pressure & lower temperatures. The H2/CO produced can be further converted to liquid hydrocarbon fuels using Fischer-Tropsch synthesis.
Since the research in thermochemical splitting of CO2/H2O is limited by the suitable material requiring high thermochemical yield, faster kinetics and near complete percentage re-oxidation yield. Therefore, this research intends to report amongst the various novel perovskite compositions based on Sr doped Praseodymium-manganite-ferrite perovskite Pr0.4Sr0.6Mn1-yFeyO3 (y= 0.2, 0.4, 0.6, 0.8, 1) the composition with highest yield for solar fuel (H2/CO) production via thermochemical splitting of H2O/CO2. Since plenty of literature is available on doping various non-redox active dopants at B-site such as Al & Ga, this novel study focuses on investigating the cation synergy by doping redox active b-site cation Mn with another redox active b-site cation as Fe on thermochemical CO2 splitting yield.
Materials & methods
The perovskites were synthesized using a modified Pechini method based on the citrate Sol-gel technique. Stoichiometric amounts of metal nitrate precursors were combined with anhydrous citric acid in a 1:1.5 molar ratio and dissolved in 100 mL of deionized water. Ethylene glycol was then added to facilitate polymerization. The resulting gel was dried, manually crushed using a mortar and pestle, and subsequently burned at 300 °C on a hot plate. The powder was further calcined in a muffle furnace at 1400 °C for 6 hours with a heating rate of 5 °C/min.
The crystal structure of the perovskite was analyzed using powder X-ray diffraction (XRD) before and after redox studies. A PANalytical EMPYREAN diffractometer was employed with a 2θ range of 20°–80°, and the obtained patterns were compared with the ICDD database for phase identification and peak analysis. The elemental composition was verified using a Thermo Scientific iCAP6000 ICP-OES to confirm the metal cation ratios. Additionally, morphological changes before and after redox reactions were examined using a JEOL JSM-7610F scanning electron microscope (SEM). The thermochemical splitting of CO₂/H₂O was investigated through redox cycling experiments in a NETZSCH thermogravimetric analyzer (TGA). Thermal reduction was performed at 1400 °C under an argon flow (100 mL/min) for 45 minutes, while re-oxidation was conducted at 800 °C in a 40% CO₂/Argon atmosphere for 60 minutes. Changes in sample weight were correlated with the oxygen non-stoichiometry (δ) of the perovskite. Equation 3, 4, 5 and 6 show the thermal reduction yield (µmol/g), thermal oxidation yield (µmol/g), % re-oxidation yield and oxygen non-stoichiometry (δ) calculation from TG data. XPS of the sample before and after the redox cycling will be used to study the cation synergy between Mn and Fe and their subsequent effect on thermochemical yield.
Where Δmredox, Wsample, , denote the change in sample weight during redox processing, the initial sample weight charged to the TGA, Molar weight of Perovskite and O2 molecule respectively.
Results and discussions
The research is ongoing and results of the redox study will be available in future.