2025 AIChE Annual Meeting
(188h) Enhanced Molecular Sensitivity of Metastable ?-WO3: Insights from Experiments and First-Principle Calculations
Authors
Meng Yin, Tohoku University
Vlasios Mavrantzas, Particle Technology Laboratory, ETH Zurich
Ken Suzuki, Tohoku University
Andreas Güntner, ETH Zürich
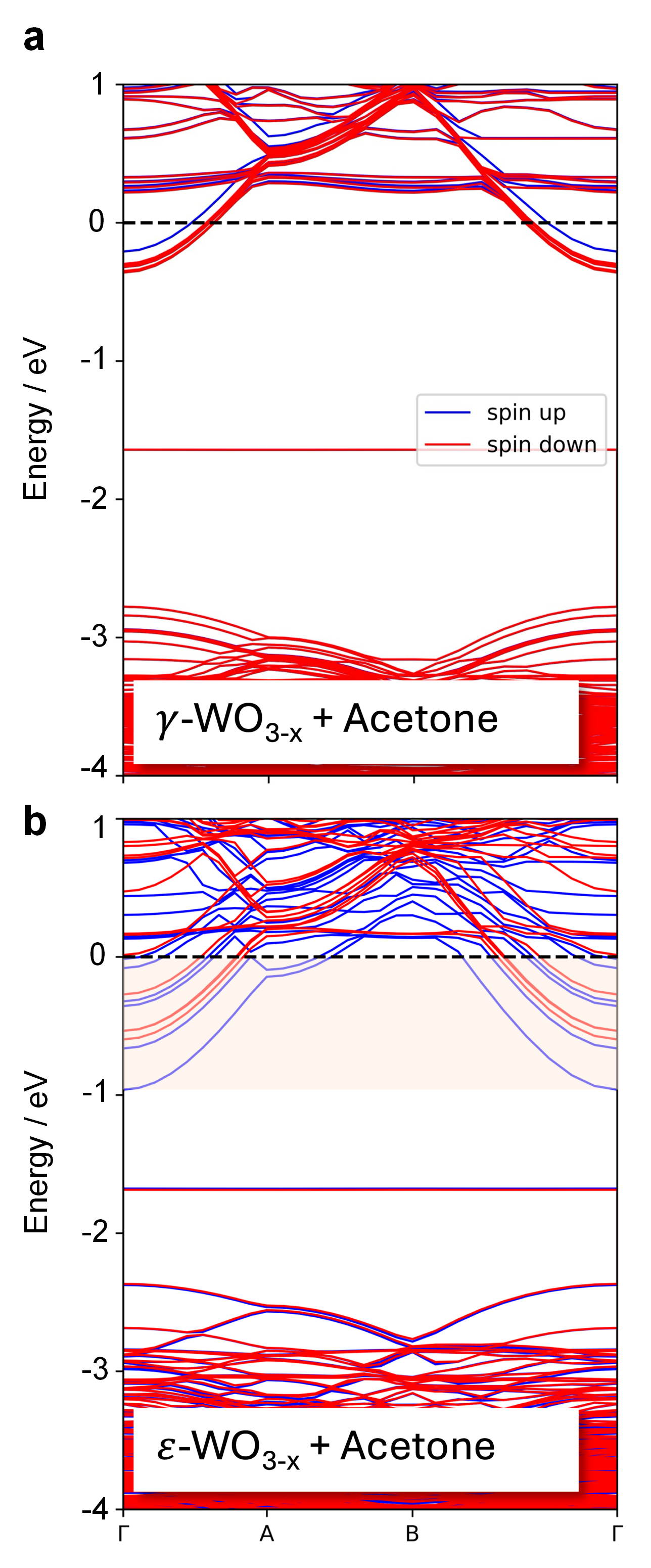
Metastable nanostructures may exhibit unique properties and outperform conventional phase-stable materials. In molecular sensing, the monoclinic ε-WO3 phase (space group Pc, #7), one of the WO3 polymorphs, has demonstrated superior acetone detection capabilities compared to its thermodynamically stable monoclinic γ-WO3 (P21/n, #14) counterpart. This has been reported since initial ε-stabilization during flame synthesis by Cr-incorporation [1], later proven to work also with Si [2]. The resultant Si-stabilized (metastable) ε-WO3 nanocrystals have been deployed for acetone detection down to a few parts-per-billion (ppb), under realistic conditions as well as in complex matrices comprising several hundreds of compounds such as human breath [3], wherein ε-WO3 sensors were also integrated with active catalyst elements to foster robustness to interferents [4]. Here, we explore the largely overlooked physico-chemical origin behind such enhanced sensor responses of the ε-polymorph, with a joint theory-experiment approach combining in situ spectroscopic analysis (IR, UV-Vis), chemisorption and operando work function (ϕ) measurements with ab initio electronic structure calculations by density functional theory (DFT). Surface chemistry and acetone interaction with the catalysts’ surface involves similar intermediates, such as acetone diolates, condensation products and carboxylate (-COO) groups in the form of acetate and formate species. Calculated adsorption energies (Eads) and overall charge transfer (Bader charges, QBader) do not differ significantly. However, our electrophysical measurements hint at enhanced electrical transduction induced by surface net-charge modulation, corroborated with band structure analysis of acetone adsorbed onto ε-WO3-x and γ-WO3-x in Figure 1. Therein, additional states within 1 eV below EF could most effectively contribute to enhanced conductivity variations of ε-WO3 when exposed to acetone. These findings highlight the limitations of surface chemistry alone (i.e., Eads, QBader) to unravel chemoresponsive behavior, that seems largely determined by energies of adsorbate-induced states.
References
[1] Wang, L; Teleki, A.; Pratsinis, S.E. & Gouma, P.I., Chem. Mater. 2008, 20, 4794 – 4796.
[2] Righettoni, M.; Tricoli, A. & Pratsinis, S.E., Chem. Mater. 2010, 22, 3152 – 3157.
[3] Güntner, A.T.; Weber, I.C.; Schon, S.; Pratsinis, S.E. & Gerber, P.A., Sens. Actuators, B 2022, 367, 132182.
[4] Weber, I.C.; Braun, H.P.; Krumeich, F.; Güntner, A.T. & Pratsinis, S.E., Adv. Sci. 2020, 7, 2001503.
Figure 1 Caption: Electronic band structures of (a) γ-WO3 and (b) ε-WO3 (001) slabs with an adsorbed acetone molecule. The pink-shaded area in (b) highlights the appearance of additional states in the energy range within 1 eV below EF.