2025 AIChE Annual Meeting
(39d) Enhanced K2CO3-Based Particle Sorbents for CO2 Capture and Regeneration
Authors
Potassium carbonate, K2CO3, was selected as a sorbent since it has the largest CO2 capture capacity of the alkali metal carbonates at 7.25 mmol CO2/g. However, the tendency of these metal carbonate sorbents to dissolve when exposed to humid environments limits their industrial scalability and recyclability (Green, et al. 2002). In this investigation we propose an enhanced K2CO3-based sorbent using bentonite clay as a binding support for increased sorbent strength and Al2O3 to improve mesopore surface area and reactivity.
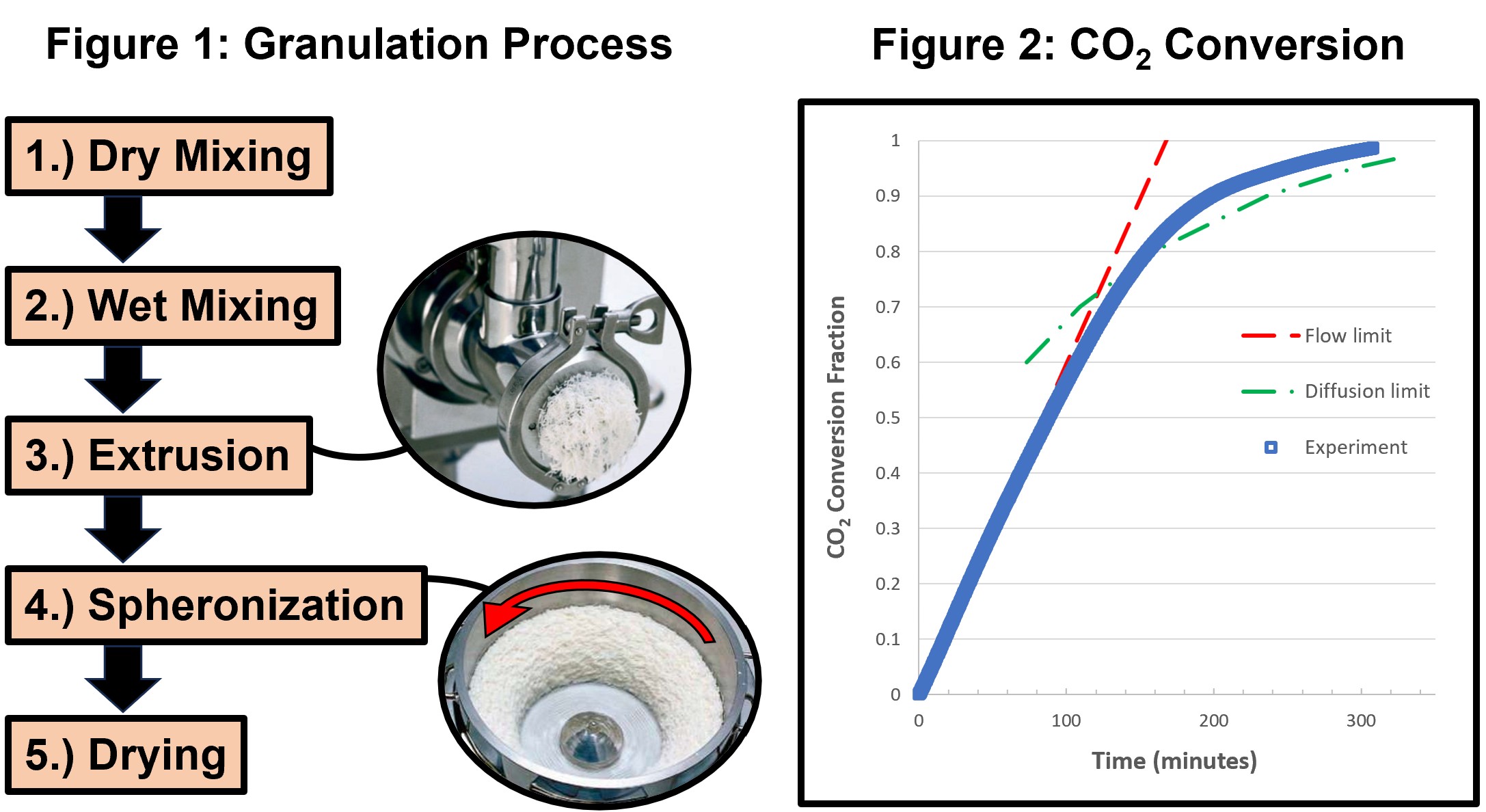
The granulation method of extrusion spheronization was chosen for its ease of pelletization and scalability (Muley, et al. 2016). The granulation process, as shown in figure 1, involved multiple steps starting with the dry and wet mixing of the raw materials, followed by extrusion and spheronization, and ending with the sorbent drying process. The drying process included superficial drying of the sorbents at 180 °C and a second drying step at 600 °C to calcine the bentonite clay support. The extruder was equipped with a 2 mm die and the sorbents were sieved to 1.4-2.8 mm.
Adsorption tests were performed in a bench-scale packed bed reactor, loaded with approximately 25 grams of sorbent. The inlet consisted of 90% Ar plus 10% CO2 by volume. The inlet stream at approximately 65 °C was humidified prior to entering the reactor; the composition of the outlet was monitored to determine the mass of CO2 captured over the experiment. Figure 2 shows an example of the experimental results in terms of the fraction of CO2 converted (normalized by the theoretical maximum CO2 based on the mass of sorbent) as a function of time. The figure includes two theoretical lines for the flow limit and the diffusion limit. At early times, the fractional conversion depends linearly on the rate at which the CO2 flows into the reactor (the flow limit line). As the reaction proceeds, a surface shell of KHCO3 begins to form that is less porous than the sorbent; as a result, the CO2 and H2O must diffuse through the surface layer to react with K2CO3. The line for the diffusion limit is based on a model of quasi-steady diffusion in a non-catalytic, shrinking core solid reactant (Wen 1968). The flow and diffusion limit analyses demonstrate that the fractional conversion is initially limited by the rate that CO2 is supplied to the reactor and subsequently limited by rate of diffusion within the sorbent particles. Further analyses can refine the design and operation of the sorbents and reactor.
Extensive experimentation found that sorbents made with 50% K2CO3 and 50% bentonite clay by mass displayed low meso-porosity and BET surface area, 3.17 m2/g. The CO2 capture capacity of these sorbents decreased after 40 cycles, going from 2.82 – 1.27 mmol CO2/g. Despite lower reactivity after multiple regeneration steps, the calcined sorbents remained intact with considerable strength. Sorbents made with 50% K2CO3, 25% bentonite clay, and 25% Al2O3 displayed higher meso-porosity and BET surface area, 14.0 m2/g, and showed no decrease in CO2 capture capacity after 40 cycles, going from 3.231 – 3.751 mmol CO2/g. The low reactivity and cyclability of the bentonite clay supported sorbents can be enhanced with the addition of Al2O3. Further optimization can be conducted on the Al2O3 and bentonite clay ratio to find a balance between sorbent reactivity and attrition resistance.
References
Guo, Y., Sun, J., Wang, R., Li, W., Zhao, C. & Li, C. (2021). Recent advances in potassium-based sorbents for CO2 capture and separation: a review, Carbon Capture Sci & Tech. 100011.
Wang, P., Sun, J., Guo, Y., Zhao, C., Li, W., Wang, G., Lei, S. & Lu, P. (2019). Structurally improved, urea-templated, K2CO3-based sorbent pellets for CO2 capture, Chem. Eng. J. 374, 20-28.
Green, D., Turk, B., Portzer, J., Gupta, R., McMichael, W., Nelson, T., Gangwal, S., Liang, Y., Moore, T., Williams, M. & Harrison, D. (2002). Carbon dioxide capture from flue gas using dry regenerable sorbents, National Energy Tech. Lab., DE-FC26-00NT40923.
Muley, S., Nandgude, T. & Poddar, S. (2016). Extrusion-spheronization a promising pelletization technique: In-depth review, Asian J. of Pharm. Sci. 11, 684-699.
Wen, C.Y. (1968). Noncatalytic heterogeneous solid-fluid reaction model, Ind. Eng. Chemistry 60, 34-54.