2025 AIChE Annual Meeting
(35b) Engineering Surface Charges of Nanofiltration Membranes to Maximize Li+/Mg2+ Separation Properties
Authors
Erda Deng, University At Buffalo
Aubrey Quigley, Purdue University
Lingxiang Zhu, National Energy Technology Laboratory
Jada Mowatt, University at Buffalo, The State University of New York (SUNY)
Benny D. Freeman, The University of Texas at Austin
Haiqing Lin, University of Buffalo, State University of New Yor
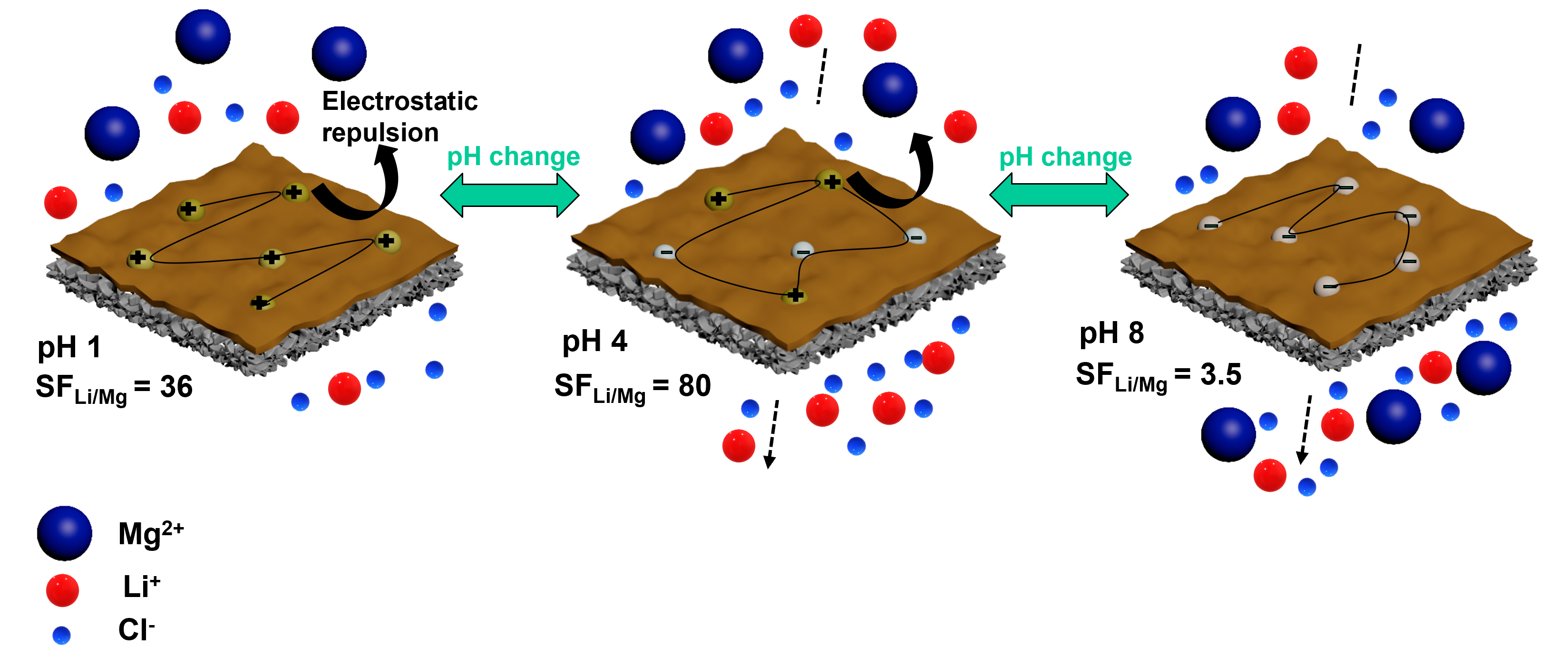
Polyamide-based nanofiltration (NF) membranes are attractive for Li+/Mg2+ separation for lithium recovery from salt brines (containing mainly Mg2+). However, Li+ and Mg2+ have similar hydration radii, leading to a low separation factor (SFLi/Mg) in state-of-the-art commercial polyamide-based nanofiltration (NF) membranes. Herein, we show that the SFLi/Mg can be dramatically enhanced by optimizing the membrane surface positive charges, achieving a maximum SFLi/Mg at a solution pH slightly below its isoelectric point (IEP). Specifically, NF270 membrane was surface-grafted by 2-(methacryloyloxy)ethyltrimethylammonium chloride (META) or polyethylenimine (PEI), assisted by bio-adhesive dopamine, forming a thin, stable, charged layer (20 – 40 nm) on the surface. The effect of the surface modification and solution pH values on the surface zeta potential (ZP) and single- and mixed-salts SFLi/Mg properties is thoroughly investigated. For instance, co-depositing META with PDA increases the ZP from 9.2 to 16 mV and increases SFLi/Mg by 130% from 34 (for NF270) to 80 at pH = 4 when treated by a salt solution (2000 ppm) with a Mg2+:Li+ mass ratio of 5.0, surpassing the state-of-the-art NF membranes. Mixed salt SFLi/Mg values are slightly higher than the single-salt values because the unbalanced Cl- ions pull smaller Li+ ions in the permeate side, further reducing their rejection, while Mg2+ rejection remains high. For instance, Li+ rejection drops from 40% to 20%, while Mg2+ rejection remains >99% at pH=4. The membrane exhibits stable separation properties over 30-h with mixed salt solutions, showcasing its potential for practical applications. This surface modification occurs at ≈22 ℃ in aqueous solutions, and it can be used to enhance commercial membrane modules for practical applications.