2025 AIChE Annual Meeting
(541e) Engineering Redox-Responsive Coacervates with Tunable Stability and Reactivity for Targeted Drug Delivery Systems
Methods: A patent-pending modification was used to crosslink spermine, introducing a novel redox-sensitive linkage. The degree of crosslinking was controlled by varying the molar ratio of spermine to modifier (1:0.125, 1:0.25, and 1:0.5). Modified spermine products were analyzed using liquid chromatography coupled with high-resolution mass spectrometry (LC-HRMS) and supported by Proton Nuclear Magnetic Resonance (1H NMR) spectroscopy. The impact of the modification on spermine’s ability to form coacervates was examined by mixing different concentrations of modified spermine (with varying degrees of crosslinking) with a fixed concentration of polyuridylic acid (polyU), an oligonucleotide. Coacervation was quantified through turbidity measurements and visualized using phase contrast microscopy. The coacervates were further characterized by dynamic light scattering (DLS) to determine their average size and size distribution. The influence of stabilizing agents, specifically poly(ethylene glycol) (PEG-6000) and PEG-diamine-6000, on coacervate longevity was also evaluated. These stabilizers were incorporated during coacervate formation, and their effect on coacervate stability across various spermine modification extents was assessed by monitoring turbidity and phase contrast images over a 24-hour period. Additionally, the redox-responsivity of the coacervates was evaluated by exposing them to reactive oxygen species (ROS), specifically hydrogen peroxide (H₂O₂), and monitoring changes in coacervate stability and structural properties. Redox-responsivity was assessed across different concentrations of H₂O₂, spermine crosslinking extents, and stabilizer types, with turbidity changes quantified and phase contrast microscopy used for visualization.
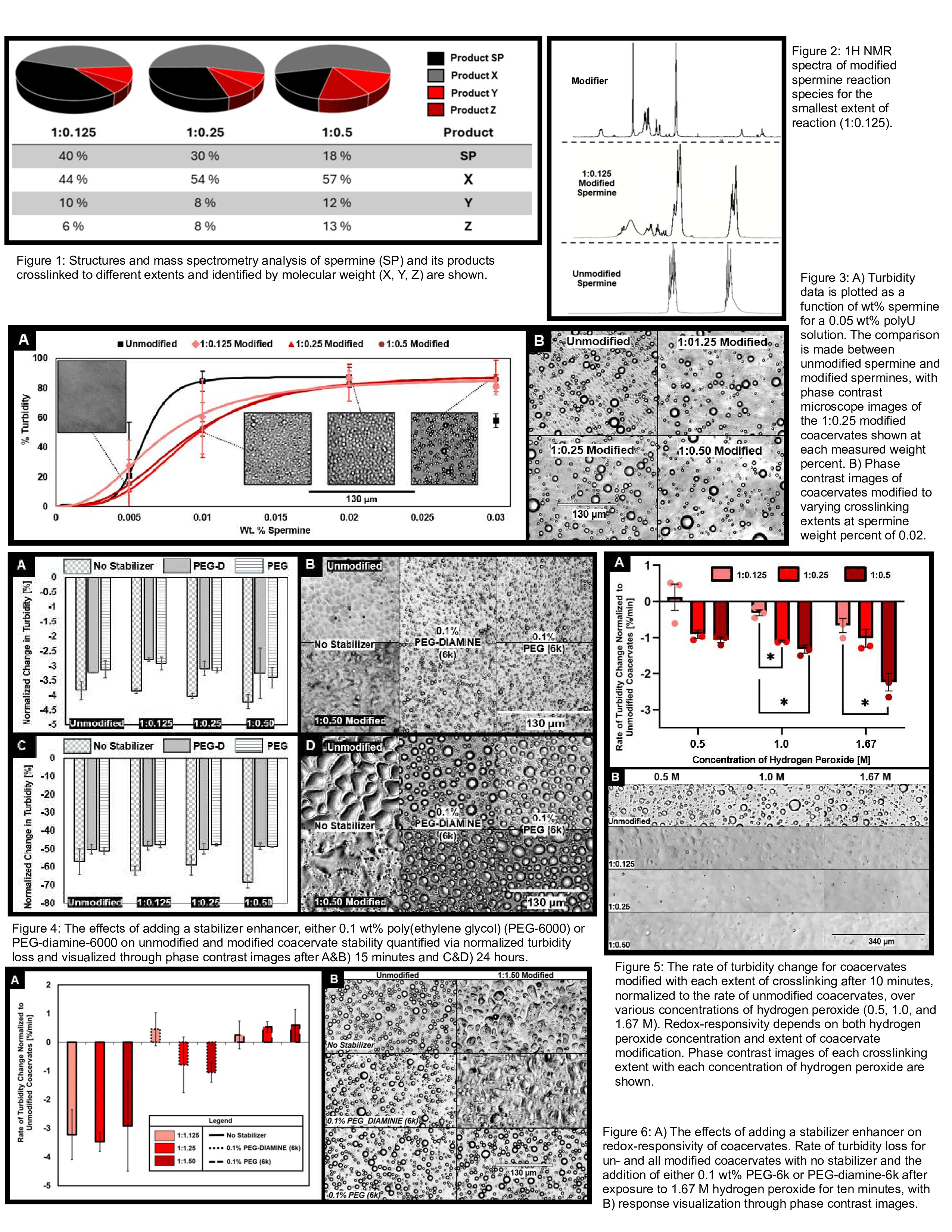
Results: The crosslinking of spermine successfully achieved, with the extent of crosslinking depending on the spermine:modifier molar ratio. LC-HRMS analysis of reaction products at ratios of 1:0.125, 1:0.25, and 1:0.5 revealed a decrease in the abundance of the unmodified spermine as modifier concentration increased, indicating effective crosslinking (Figure 1). The crosslinked products were identified by their molecular weights, and the abundance of each product increased with higher modifier concentration, confirming a direct correlation between modifier concentration and the formation of larger, more complex crosslinked structures. Additionally, 1H NMR spectroscopy confirmed the chemical structure of the modified spermine, with characteristic shifts indicating the formation of the redox-sensitive linkage (Figure 2). To assess the impact of spermine modification on coacervation, we first established the baseline by determining the minimum concentration of unmodified spermine required to coacervate with polyU (≈80%) at approximately 0.01 wt%, consistent with previous literature. Modified spermine concentrations ranging from 0.005 wt% to 0.03 wt% were then tested, and coacervation of polyU with modified spermine was observed at all molar ratios, with a minimum concentration of approximately 0.02 wt% for all modification levels (Figure 3). These results indicate that the modification had a negligible effect on spermine's charge and its ability to form coacervates with polyU. DLS measurements showed that the average particle size of coacervates was not significantly affected by the extent of spermine crosslinking. Unmodified spermine coacervates had an average diameter of 1623.33 ± 81.71 nm, while coacervates formed with spermine modified to the highest extent (1:0.5) had a similar average diameter of 1556.89 ± 202.33 nm. The polydispersity index (PDI) for unmodified coacervates was 0.319 ± 0.254, and for 1:0.5 modified coacervates, it was 0.232 ± 0.136, indicating minimal impact on size distribution and particle uniformity due to the modification. The stabilizing effect of PEG-6000 and PEG-diamine-6000 was assessed, with both stabilizers improving coacervate stability over non-stabilized coacervates after both 15 minutes and 24 hours. Turbidity measurements showed only slight differences, with PEG-diamine-6000 and PEG-6000 coacervates exhibiting a slightly slower loss of turbidity compared to their non-stabilized counterparts for each crosslinking ratio (Figure 4). Phase contrast microscopy shows both PEG-6000 and PEG-diamine-6000 maintained coacervation more effectively than the non-stabilized coacervates with minimal aggregation and clearer phase separation observed. Redox-responsivity of the coacervates was evaluated by exposing coacervates of each crosslinking ratio to varying concentrations of hydrogen peroxide (H₂O₂) and monitoring changes in coacervate stability and structural properties. The rate of turbidity change, reflecting coacervate phase separation, was dependent on both the concentration of H₂O₂ and the extent of spermine crosslinking (Figure 5). Coacervates with modified spermine exhibited significantly faster dissociation in response to the presence of H₂O₂ compared to unmodified coacervates. The rate of turbidity loss increased with both higher concentrations of H₂O₂ and greater crosslinking extents, after 10 minutes of exposure. Phase contrast microscopy further confirmed these findings, revealing significant structural loss in each of the modified coacervates, whereas the unmodified coacervates maintained their structure even after H₂O₂ exposure. The impact of stabilizers on redox-responsivity was evaluated by testing unmodified and all modified coacervates at the highest hydrogen peroxide concentration, both with and without each stabilizer. Turbidity measurements and phase contrast microscopy revealed distinct differences in structural integrity (Figure 6). Coacervates stabilized with PEG-DIAMINE-6000, as well as unmodified coacervates, exhibited a similar degree of redox-responsivity, though slightly reduced in the presence of the stabilizer, with clear dissociation observed upon exposure to hydrogen peroxide. In contrast, coacervates stabilized with PEG-6000 retained phase separation and showed no significant structural deformation, suggesting that PEG-6000 interfered with the coacervates’ ability to respond to oxidative stress.
Conclusions: Complex coacervation offers significant potential for the design of responsive delivery systems that can dynamically react to environmental cues. In this study, we explore the creation of redox-responsive coacervates by modifying spermine through a novel reaction, enabling the fine-tuning of their response to oxidative stress. By modifying spermine, we were able to create coacervates with tunable redox-responsivity, where the extent of spermine crosslinking directly influenced their reaction to oxidative stress. This enables precise control over the coacervate's stability and responsiveness to environmental changes. The findings underscore the ability to engineer coacervates with variable properties by adjusting the crosslinking degree, which could be leveraged for applications requiring responsive behavior under specific conditions. Additionally, our results demonstrate the critical role of stabilizing agents in maintaining coacervate integrity. While both PEG-6000 and PEG-DIAMINE-6000 enhance stability, only PEG-DIAMINE-6000 preserves the redox-responsivity of the coacervates, highlighting the importance of selecting appropriate stabilizers for systems that must retain their functional responsiveness. PEG-6000, while stabilizing the coacervates, hindered their ability to undergo structural changes in response to oxidative stress, which could limit its use in systems where environmental reactivity is essential. Overall, this work contributes to the growing understanding of how to engineer coacervates with adaptable properties, offering new possibilities for responsive materials in a variety of applications, including drug delivery and adaptive materials design.