2025 AIChE Annual Meeting
(3ix) Engineering Polymer and Ceramic Interfaces for Improved Li+ Transport in Composite Solid Electrolytes
Beyond Li-ion Batteries; Polymer Recycling; CO2 Capture; Electrochemistry; Advancing Materials Characterization Techniques
Abstract:
Solid-state electrolytes are a promising field of study to improve the safety and energy density of lithium-based batteries. Composite solid electrolytes seek to leverage both the desirable mechanical properties of polymers and the high conductivity of active ceramic fillers; however, Li+ transport through the composite, and especially the extent of Li+ through the ceramic phase, remains an open question.1, 2
Simulations suggest that decreasing interfacial resistance between polymer and ceramic would promote transport through ceramic particles.1 Our work seeks to improve Li+ transport in the ceramic phase by engineering both polymer and ceramic materials that minimize the interfacial resistance between the two.
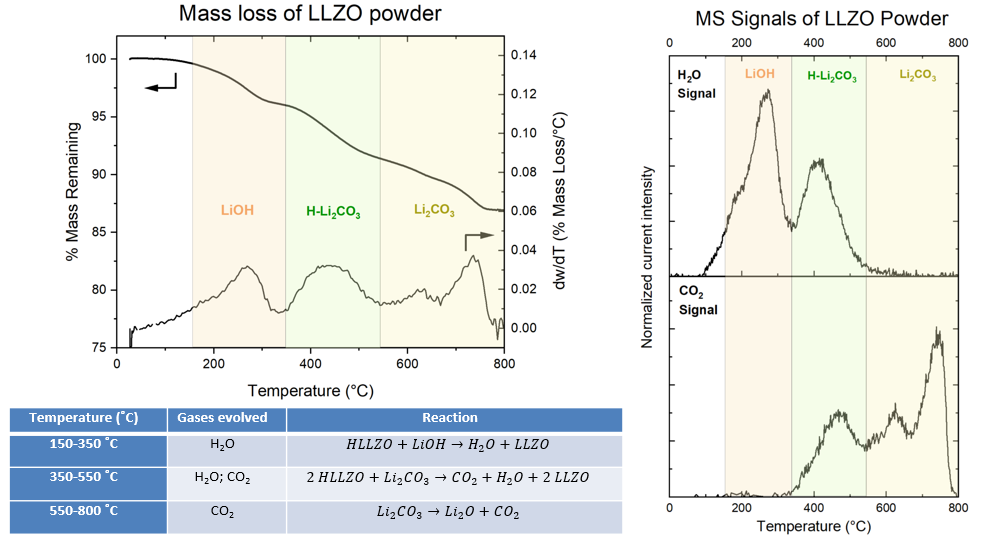
We consider how lithium carbonate (Li2CO3) and lithium hydroxide (LiOH) content on the surface of LLZTO (Li6.4La3Zr2Ta0.6O12) affects Li+ transport. Accompanying this study is a detailed description of sintering procedures used to clean the LLZTO surface. Titration Mass Spectrometry (TiMS) is used to quantify Li2CO3 content in our ceramic particles and assess the effectiveness of our cleaning procedures. Thermogravimetric analysis mass spectrometry (TGA-MS) was employed to identify key temperatures for targeted removal of specific contaminants. We identified 3 unique contaminant regimes, one of LiOH and two of Li2CO3. Li2CO3 exhibits two unique regimes depending on its contact with protonated LLZO. These regimes decompose at different temperatures and result in the release of different gases, as is shown in the Figure below.
Traditional polymer electrolytes have a low transference number (~0.15) compared to ceramics (~1), and when combined in composites this mismatch has been suggested as a major contributor to interfacial impedance.3 We explore the effect of the transference number mismatch by using a single-ion conducting polymer poly((trifluoromethane)sulfonamide lithium methacrylate) (PMTFSILi). The Li+ transport and electrochemical performance in a composite electrolyte with high transference number PMTFSILi is compared with that of a system utilizing a lithium salt.
- Kim, HK., Barai, P., Chavan, K. et al.Transport and mechanical behavior in PEO-LLZO composite electrolytes. J Solid State Electrochem 26, 2059–2075 (2022). https://doi.org/10.1007/s10008-022-05231-w
- Zheng J., Hu, Y. New Insights into the Compositional Dependence of Li-Ion transport in polymer-ceramic composite electrolytes. ACS Appl. Mater. Interfaces, 10, 4, 4113–4120 (2018). https://doi.org/10.1021/acsami.7b17301
- Mehrotra A. et al. Quantifying Polarization Losses in an Organic Liquid Electrolyte/Single Ion Conductor Interface. Electrochem. Soc. 161 A1681 (2014). https://doi.org /10.1149/2.0721410jes