2025 AIChE Annual Meeting
(432h) Engineered Hydrogel Biomaterial Resists the Foreign-Body Response
Authors
A novel hydrogel, Amikagel (AM) based platform is developed, derived from aminoglycosides. It is a polymer of amikacin hydrate and poly (ethylene glycol) diglycidyl ether (PEGDE) generated by emulsion polymerization reaction at 40℃ for 8 h. Different stochiometric ratios of amikacin hydrate and the cross-linker PEGDE resulted in distinct viscoelastic properties of Amikagel and 3 different Amikagels (AM2, AM5 and AM7). A 1-2 cm midline incision was created along the spinal cord, and a small pocket is being made, in which a 5 mm Amikagel biomaterial is inserted. As a positive control, a PEG2000 hydrogel was synthesized by mixing PEGDA in H2O with photoinitiator 2-Hydroxy-4'-(2-hydroxyethoxy)-2-methylpropiophenone (I295). The biocompatibility of the biomaterials was tracked for 28 days, and skin tissues are collected to perform immunohistochemistry to study immune cell markers (F4/80, Arginase-1) and fibroblast activation (α-sma).
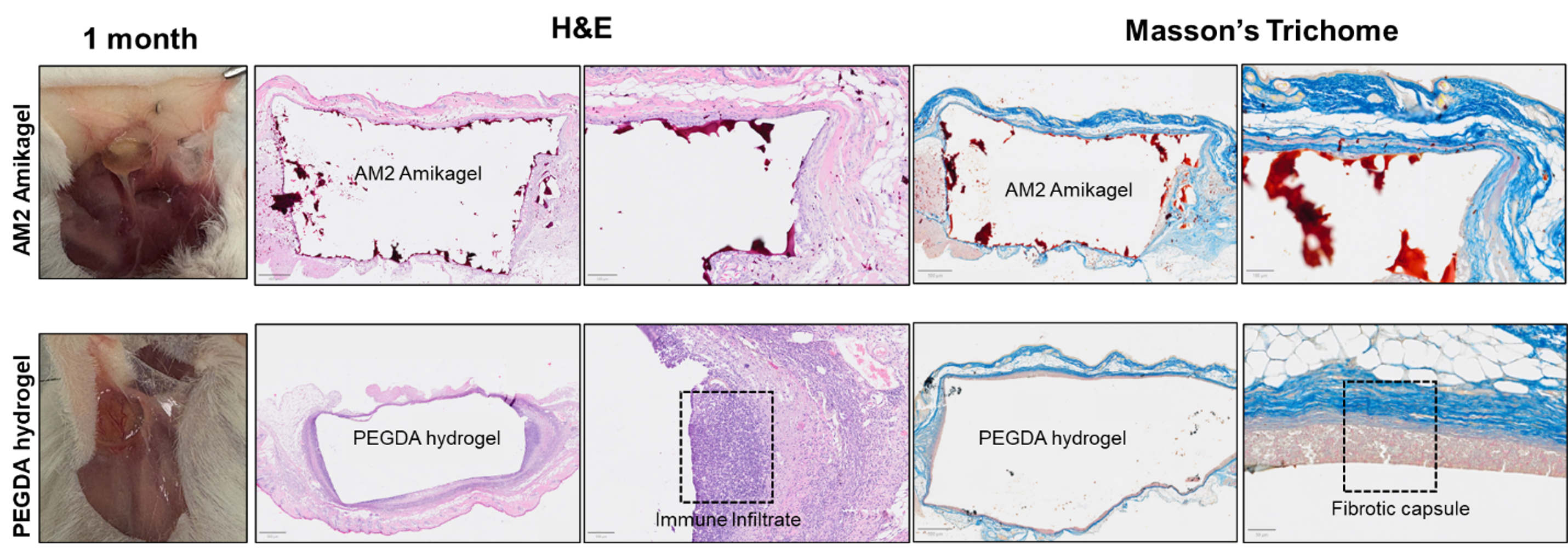
Based on the biocompatibility study, we observe tissue ulceration in AM5 and AM7 groups by day 7, but no adverse effects or inflammation was observed in AM2 group by day 28. From Masson’s Trichome Staining, collagen deposition was not observed in AM2 groups whereas there were a lot of aligned collagen at the interface of hydrogel and skin and influx of inflammatory cells from skin to PEGDA hydrogel. Further investigation into their immunohistochemistry profile, α-sma % positive cells were higher in AM2 hydrogel, signifying the nutrients exchange around the biomaterial, which significantly reduces the chances of foreign body response (FBR). Inflammatory cell thickness is significantly lesser (3-fold) in AM2 compared to positive control. No difference in expression was observed in pro-inflammatory macrophages, whereas pro-resolution macrophages (Arginase-1) positive cells were lesser in AM2 group.
In summary, we designed a biomaterial with an anti-FBR property and low collagen deposition which could attribute to its use in wide applications in implantable biomaterials and biomedical devices.
Figure 1: Staining images of AM2 and PEGDA hydrogels after being subcutaneously implanted in mice for 28 days.