2025 AIChE Annual Meeting
(584g) Elucidating the Operational Degradation of Ni–Mo Composites Towards Alkaline Hydrogen Evolution
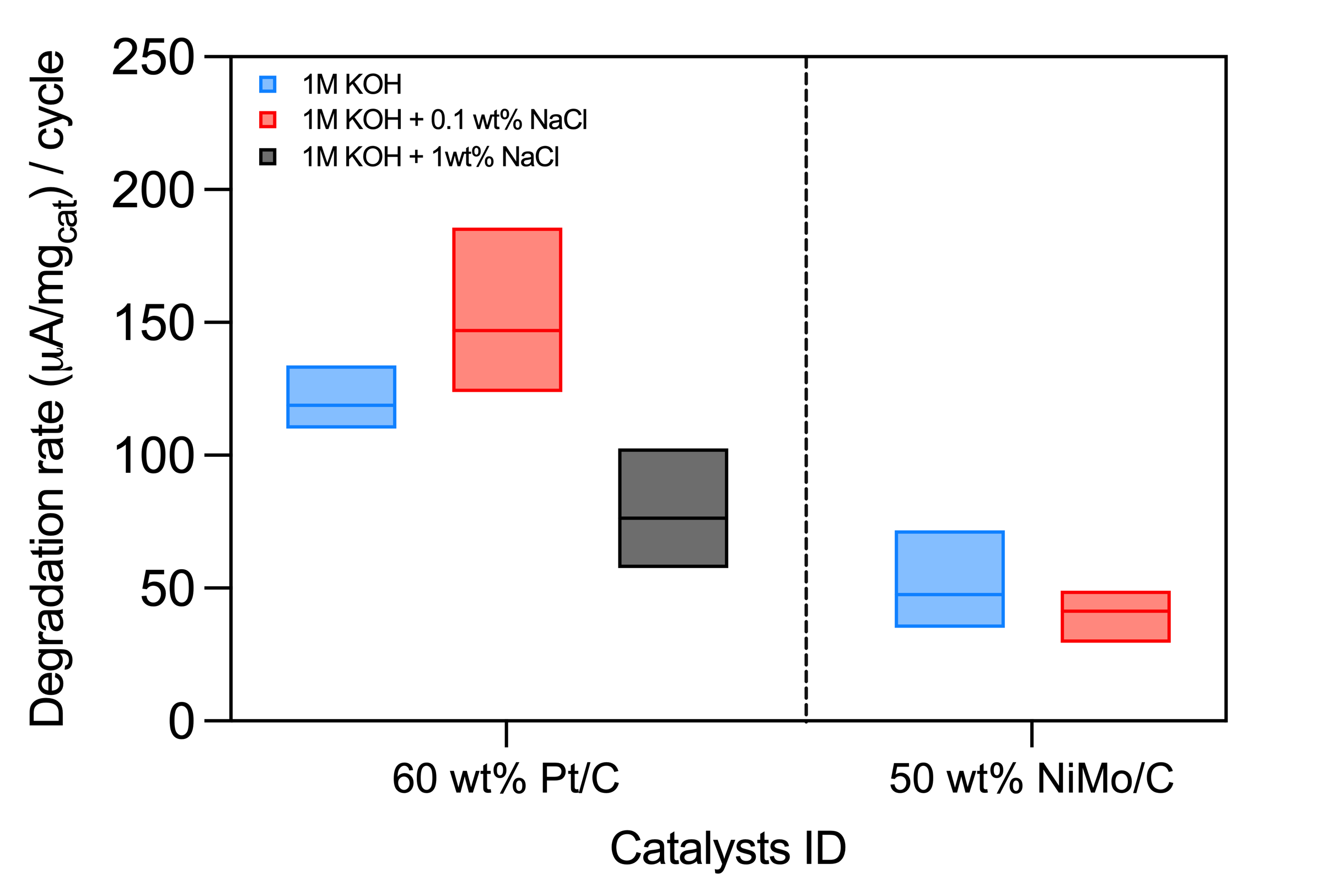
We have observed that Ni–Mo composites demonstrate superior stability in alkaline electrolytes containing chloride salts compared to platinum, as shown through accelerated stress tests using a thin-film rotating disk electrode setup. For example, incorporation of NaCl at 0.1 wt% in 1 M KOH electrolyte leads to a ~25% increase in the degradation rate—measured as the cathodic current density at a fixed iR-free overpotential—compared to pure 1M KOH control. By contrast, Ni–Mo composites exhibit markedly slower decrease in HER current in saline electrolytes.
Ongoing work focuses on evaluating these electrocatalyst using a half-cell gas diffusion electrode assembly4 to better assess electrolyzer cathode durability realistic operating conditions. These findings highlight the promise of Ni–Mo composites in terms of both material stability and durability, reinforcing their potential as competitive catalysts for the alkaline HER.
Reference:
- Zeng & Li, J. Mater. Chem. A, 2015, 3, 14942–14962.
- Raj, J. Mater. Sci., 1993, 28, 4375–4382.
- Nairan et al., Adv. Funct. Mater., 2019, 29, 1903747.
- Jiménez et al., J. Mater. Chem. A, 2023, 11, 20129–20138.