2025 AIChE Annual Meeting
(584an) Elucidating the Effect of Charge Compensating Species on Aluminum-Oxygen Bond Cleavage in the Dissolution of the Faujasite Framework
Authors
Charles Umhey - Presenter, Washington State University
Zheng Cui, Tulane University
Daniel Shantz, Tulane University
Jean-Sabin McEwen, Washington State University
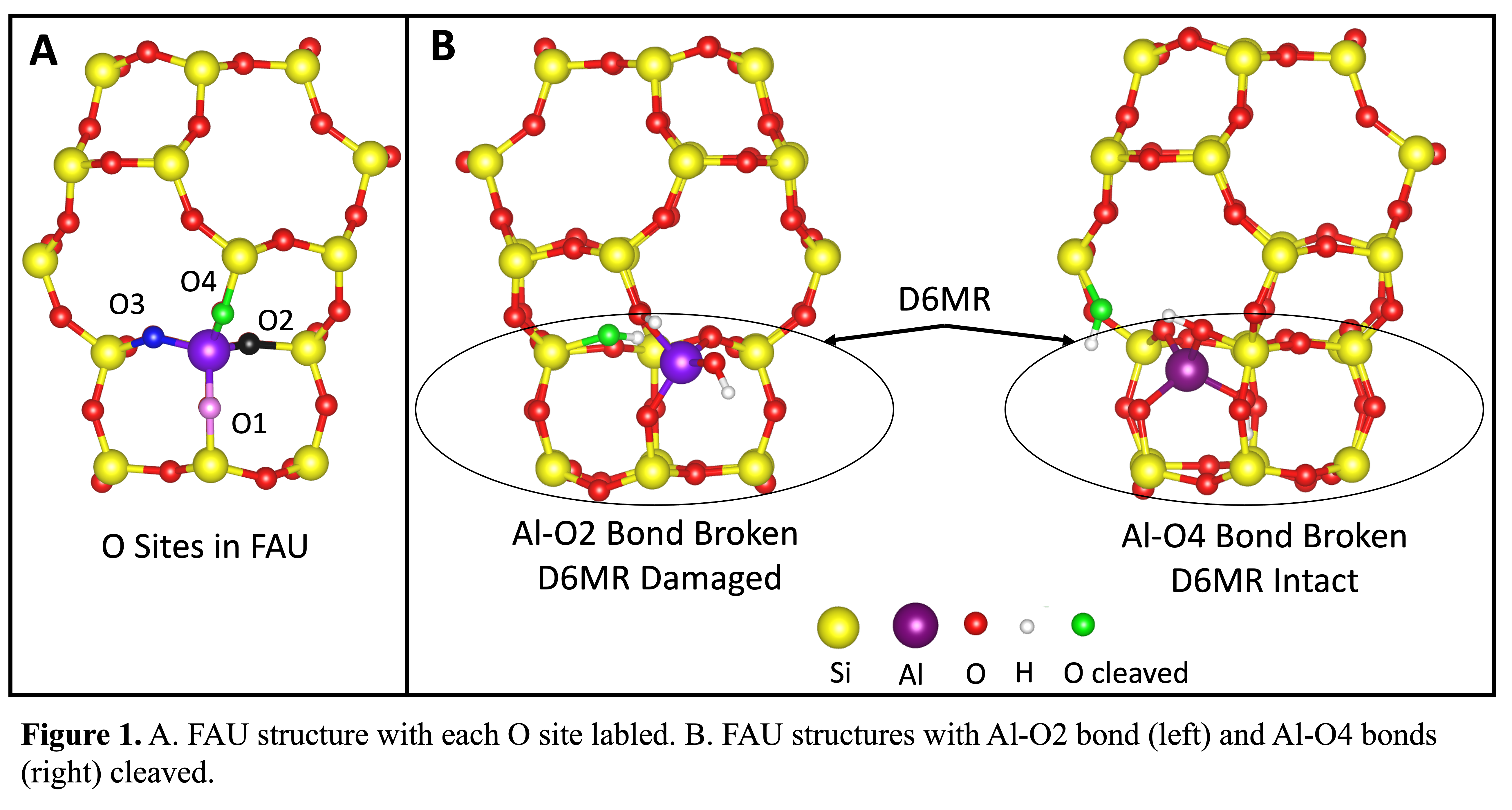
In the synthesis of SSZ-39, The faujasite framework (FAU) is often used as both a silicon and an aluminum source. Due to the presence of double six membered rings in both SSZ-39 and FAU it has been suggested the double six membered rings (D6MRs) remain intact during synthesis and reconnect to form SSZ-39. If D6MRs remain intact this could lead to faster synthesis and would mean product aluminum distributions would be heavily influenced by parent aluminum distributions. In FAU there are 4 symmetrically distinct Al-O bonds (see Figure 1A), only one of which, the Al-O4 bond, can be broken without damaging the D6MR. This presents an opportunity to evaluate the feasibility of preserving D6MRs during synthesis since Al-O4 bonds should be more favorable to break than other Al-O bonds for D6MR subunits to remain intact. Using DFT calculations we compare the thermodynamic and kinetic favorability of cleaving Al-O4 bonds (see Figure 1B) compared to the favorability of cleaving other Al-O bonds to determine if the preservation of D6MRs in the interconversion of FAU to other zeolite frameworks is realistic. Reaction barriers were found to be sensitive to the Al-O bond being broken, with cleaving the Al-O4 bond having the highest reaction barrier of 1.63 eV and cleaving the Al-O2 bond having the lowest reaction barrier of 0.83 eV. These results suggest the D6MRs are not preserved during the interconversion of FAU to other zeolites since the Al-O4 bond is the only bond that can be cleaved without damaging the D6MR and it is the most difficult bond to cleave. Preliminary results with spectator species such as sodium atoms and organic structure directing agents show dealumination barriers are also sensitive to the chemical environment during dealumination, suggesting dealumination pathways can be controlled by modifying the chemical environment during zeolite interconversion.