2025 AIChE Annual Meeting
(590t) Elucidating catalyst-ionomer contact in anion exchange membrane water electrolysis
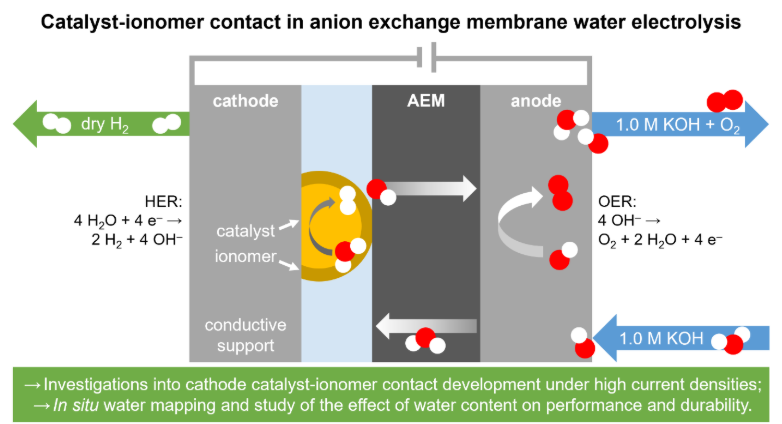
Recent work by Kong et al. has shown that the cathode plays the main role in early stage degradation of AEMWE systems.1 Migration of Pt catalyst particles was identified as the largest contributor to the degradation of the cathode. Although operation without catholyte reduced this problem slightly, a significant amount of Pt agglomeration was nevertheless observed, emphasizing that the degradation of dry cathodes in AEMWE is still problematic. The use of an ionomer as binder for the Pt particles, although beneficial, does not fully solve this issue. Additionally, the ionomer:catalyst ratio needs to be tuned carefully, as too high concentrations of binder can block the catalytically active sites.
Apart from binding the hydrogen evolution reaction (HER) catalyst particles to the conductive support, the ionomer is also applied to facilitate easy transport of hydroxide ions from the cathode to the AEM. Weakened interaction between the catalyst and ionomer can lead to the formation of small pockets between the two interfaces when hydrogen gas is generated, hindering the transport of electrons and hydroxide ions and thus resulting in increased ohmic resistances. Additionally, the migration of Pt particles is expected to be more pronounced when the contact between the ionomer and catalyst is disrupted.
In this work, the interaction between the ionomer and catalyst is investigated under high current densities. These circumstances are known to lead to inhomogeneous water distribution at the cathode,2 which is hypothesized to cause local stress in the catalyst layer. Swelling and deswelling of the ionomer could lead to weakened contact between the catalyst and ionomer, which is expected to induce severe degradation issues. We investigate the swelling behavior of the ionomer in relation to the relative humidity at the cathode by mapping of the local pressure built-up in the cell during operation. We also quantify the Pt migration via TEM and XPS, where the latter is also used to detect possible migration of the ionomer. In a later stage of this work, these results can be coupled to in situ neutron imaging to dynamically investigate the water content in the cell under operation.
Keywords: Electrochemical fundamentals, Energy (Sustainability & Environment), Carbon management
References:
(1) Kong, T.-H.; Cha, J.; Lee, H.; Park, N.; Kwon, S.; You, H.; Cha, S. G.; Kwon, Y. A Cathode Is the Key Contributor to the Initial Degradation of Anion Exchange Membrane Water Electrolyzers. ACS Energy Lett. 2025, 3647–3654. https://doi.org/10.1021/acsenergylett.5c01785.
(2) Koch, S.; Disch, J.; Kilian, S. K.; Han, Y.; Metzler, L.; Tengattini, A.; Helfen, L.; Schulz, M.; Breitwieser, M.; Vierrath, S. Water Management in Anion-Exchange Membrane Water Electrolyzers under Dry Cathode Operation. RSC Adv. 2022, 12 (32), 20778–20784. https://doi.org/10.1039/D2RA03846C.