2025 AIChE Annual Meeting
(657b) Electromagnetic Ptsn Thermocatalysis for Energy-Efficient Upcycling of Waste Plastics to Olefins
Authors
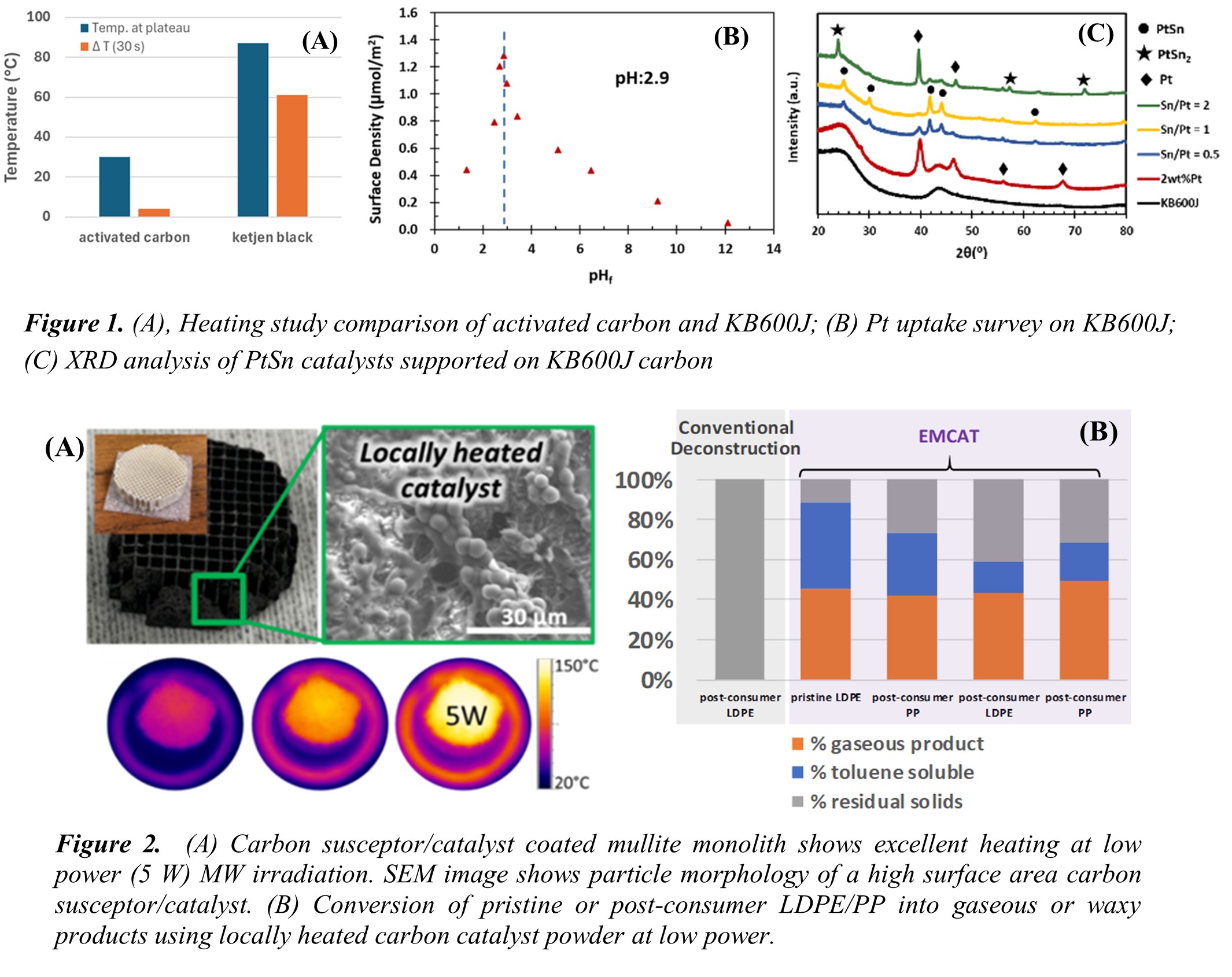
Semi-graphitic carbon materials were employed as microwave (MW) susceptors due to their strong electromagnetic (EM) heating response. As shown in Figure 1A, the selected carbon support, Ketjen Black 600JD, exhibits significantly higher MW absorption and temperature conversion efficiency compared to conventional carbon materials. Figure 1B highlights the metal uptake behavior on the carbon support, revealing an optimal pH of 2.9 for precursor adsorption. Utilizing this method, monometallic Pt/KB600J was synthesized through strong electrostatic adsorption, and bimetallic catalysts PtSnx/KB600J were synthesized via the SEA and Sn wetting approach. X-ray diffraction (Figure 1C) confirms the formation of alloy and intermetallic phases upon incorporation of a secondary metal, with clear phase evolution observed as the composition varies.
Figure 2A illustrates the morphology of in-house synthesized meso-graphitic carbon coated onto a mullite monolith, which exhibits strong microwave (MW) heating efficiency at low power input. This setup enabled an effective low-temperature, MW-assisted thermocatalytic process for plastic deconstruction. As shown in Figure 2B, the process achieved rapid conversion of plastic feedstocks LDPE and PP into low- and medium-molecular-weight hydrocarbons, with significantly reduced energy consumption and reaction times compared to conventional thermal methods. Notably, MW-assisted reactions yielded higher product yields at significantly lower temperatures and shorter durations, whereas conventional heating under similar conditions resulted in minimal conversion.
This work demonstrates significant advancement in microwave-assisted catalytic plastic upcycling by developing bifunctional PtSnx catalysts supported on microwave-active carbon materials such as KB600J and meso-graphitic carbon (MGC). When coated on high-surface-area monoliths, these catalysts enabled low-temperature, energy-efficient thermocatalytic deconstruction of LDPE and PP, achieving up to 89% conversion into low and medium-molecular-weight hydrocarbons with a ~80% reduction in energy consumption compared to conventional pyrolysis. These findings highlight the potential of EM-coupled PtSnx/carbon systems as a scalable and sustainable platform for the valorization of plastic waste.