2025 AIChE Annual Meeting
(22e) Electrochemical Sensing of Neuropeptide Y Using Peptide-Based Detection Molecule
Authors
Neuropeptide Y (NPY), the most abundant neuropeptide in the mammalian brain, plays critical roles in appetite regulation, stress response, and circadian rhythms. Dysregulated NPY levels are implicated in numerous disorders, including obesity, PTSD, depression, and cancer. However, detecting NPY remains challenging due to its extremely low concentrations in vivo, often in the picomolar range. Conventional methods such as HPLC-MS and fluorescence immunoassays offer limited sensitivity or require labor-intensive workflows. Electrochemical impedance spectroscopy (EIS) provides a label-free, cost-effective alternative, but most current sensors rely on aptamers or antibodies that suffer from folding instability and limited robustness in complex biological matrices. In this work, we introduce a peptide-based electrochemical sensor leveraging a synthetic biorecognition molecule, P1N2, to overcome these limitations. Peptides offer high chemical stability, low immunogenicity, and rapid binding/dissociation kinetics, making them ideal for real-time sensing. We hypothesize that peptide-based BRMs can achieve stable and selective detection of NPY even in complex neurochemical environments. Using a commercially available screen-printed gold electrode and EIS readout, our platform successfully detects NPY at picogram-per-milliliter levels, with minimal interference from dopamine, norepinephrine, and serotonin. This work provides a robust and generalizable strategy for neuropeptide sensing with future potential for point-of-care or wearable applications.
Method and Material
This study presents a peptide-based electrochemical sensing platform for NPY detection, integrating surface functionalization chemistry with electrochemical impedance spectroscopy (EIS). The core sensing element is the synthetic peptide P1N2 (CGGGYHPNGMNPYTKA), which specifically binds NPY. The platform utilizes screen-printed gold electrodes (SPEs), which were first electrochemically cleaned by cyclic voltammetry (CV) in 0.5 M H₂SO₄ (−0.3 to 1.1 V, 100 mV/s, 30 cycles) to ensure a clean surface. For immobilization, a self-assembled monolayer of 11-mercaptoundecanoic acid (MUA, 0.1 mM in ethanol) was formed on the gold surface, followed by EDC/NHS activation (4:1 in 0.2 M MES buffer, overnight at 4 °C). The peptide P1N2 was then immobilized at a concentration of 100 μg/mL in PBS for 2 hours, and 0.5% BSA was used to block nonspecific binding sites. NPY samples (10–200 pM in PBS) were applied to the functionalized electrodes and incubated for 2 hours. Electrochemical measurements were carried out using a redox probe containing 5 mM K₃Fe(CN)₆/K₄Fe(CN)₆ in 0.1 M KCl. CV was performed from −0.2 to 0.5 V (100 mV/s, 20 cycles), and EIS measurements were acquired at 0.2 V vs. Ag/AgCl with a 10 mV AC perturbation over a frequency range of 1 MHz to 0.01 Hz, enabling sensitive, label-free detection of NPY.
Results and Discussion
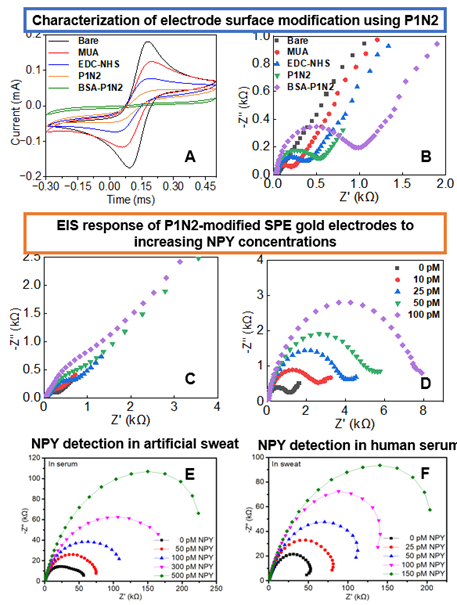
We developed a peptide-based electrochemical sensor by immobilizing P1N2 onto screen-printed gold electrodes and characterized the surface modification using cyclic voltammetry (CV) and electrochemical impedance spectroscopy (EIS). As shown in Fig. 1A, CV revealed an increased peak-to-peak separation after peptide immobilization, indicating reduced electron transfer kinetics. This was further supported by EIS analysis (Fig. 1B), where a pronounced increase in charge transfer resistance (Rct) confirmed the formation of a dense, insulating P1N2 monolayer. We then evaluated sensor performance for NPY detection under various conditions. In the absence of a redox probe, EIS showed a concentration-dependent increase in impedance upon NPY binding (Fig. 1C), consistent with the formation of P1N2-NPY complexes at the interface. Upon adding 5 mM K₃Fe(CN)₆/K₄Fe(CN)₆, the impedance response exhibited greater sensitivity and dynamic range (Fig. 1D), validating the use of a redox couple for enhanced signal resolution. To assess real-world applicability, the sensor was tested in human serum and artificial sweat. As shown in Fig. 1E, the sensor maintained strong, concentration-dependent Rct shifts in serum, while Fig. 1F shows a similar trend in sweat with slightly attenuated signals, likely due to ionic composition differences. These results highlight the sensor’s robustness and potential for non-invasive neuropeptide monitoring.
Conclusion
We developed an electrochemical sensing platform using a peptide-based biorecognition molecule, P1N2, immobilized on gold electrodes and characterized via electrochemical impedance spectroscopy (EIS). The sensor showed a strong and linear impedance response to NPY across physiologically relevant concentrations in PBS, with improved sensitivity in the presence of a redox probe. In complex media such as human serum and artificial sweat, the sensor maintained excellent linearity and functional performance. These results highlight the robustness and versatility of peptide-based sensors, supporting their potential for non-invasive, real-time neuropeptide monitoring in wearable or microneedle-integrated diagnostic systems.
Figure 1. Characterization of electrode surface modification using P1N2. Results from both CV (A) and EIS (B) tests for each step of P1N2 deposition. Both decreased redox peaks and increase in the impedance value in each step indicate successful modification electrode surface. Nyquist plots showing EIS response of P1N2-modified SPE gold electrodes to increasing NPY concentrations (0–100 pM). (C) Measurements in PBS without redox probe. (D) Measurements in PBS with 5 mM K3Fe(CN)6/K4Fe(CN)6, showing enhanced sensitivity. All tests were conducted at 0.2 V vs Ag/AgCl. Evaluation of P1N2-modified SPE gold electrodes for NPY detection in physiologically relevant media. (E) Nyquist plots showing the impedance response of the sensor in human serum at increasing NPY concentrations (0 – 500 pM). (F) Nyquist plots for NPY detection in artificial sweat, showing a similar concentration-dependent impedance response.